Votrient
Generic name: pazopanib
Drug class: VEGF/VEGFR inhibitors
Medically reviewed by A Ras MD.
What is Votrient?
Votrient is a prescription medicine used to treat people with advanced renal cell cancer (RCC), advanced soft tissue sarcoma (STS) who have received chemotherapy in the past
It is not known if Votrient is effective in treating certain soft tissue sarcomas or certain gastrointestinal tumors.
It is not known if Votrient is safe and effective in children under 18 years of age.
Description
Pazopanib is a kinase inhibitor. Pazopanib is presented as the hydrochloride salt, with the chemical name 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methylbenzenesulfonamide monohydrochloride. It has the molecular formula C21H23N7O2S•HCl and a molecular weight of 473.99 g/mol. Pazopanib hydrochloride has the following chemical structure:
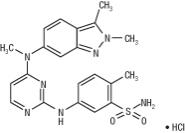
Pazopanib hydrochloride is a white to slightly yellow solid. It is very slightly soluble at pH 1 and practically insoluble above pH 4 in aqueous media.
VOTRIENT tablets are for oral use. Each 200-mg tablet of VOTRIENT contains 200 mg of pazopanib equivalent to 216.7 mg of pazopanib hydrochloride. The inactive ingredients of VOTRIENT are: Tablet Core: magnesium stearate, microcrystalline cellulose, povidone, and sodium starch glycolate. Coating: Gray or pink film-coat: hypromellose, iron oxide black, macrogol/polyethylene glycol 400 (PEG 400), polysorbate 80, and titanium dioxide.
Mechanism of Action
Pazopanib is a multi-tyrosine kinase inhibitor of vascular endothelial growth factor receptor (VEGFR)-1, VEGFR-2, VEGFR-3, platelet-derived growth factor receptor (PDGFR)-α and -β, fibroblast growth factor receptor (FGFR)-1 and -3, cytokine receptor (Kit), interleukin-2 receptor-inducible T-cell kinase (Itk), lymphocyte-specific protein tyrosine kinase (Lck), and transmembrane glycoprotein receptor tyrosine kinase (c-Fms). In vitro, pazopanib inhibited ligand-induced autophosphorylation of VEGFR-2, Kit, and PDGFR-β receptors. In vivo, pazopanib inhibited VEGF-induced VEGFR-2 phosphorylation in mouse lungs, angiogenesis in a mouse model, and the growth of some human tumor xenografts in mice.
What is the most important information I should know about Votrient?
Votrient can cause serious liver problems including death. Your healthcare provider will do blood tests to check
your liver before you start and while you take Votrient.
Tell your healthcare provider right away if you get any of these signs of liver problems during treatment with Votrient:
- yellowing of your skin or the whites of your eyes (jaundice)
- loss of appetite
- dark urine
- pain on the right side of your stomach area (abdomen)
- tiredness
- bruise easily
- nausea or vomiting
Your healthcare provider may need to prescribe a lower dose of Votrient for you or tell you to stop taking Votrient if you develop liver problems during treatment.
What should I tell my healthcare provider before taking Votrient?
Before you take Votrient, tell your healthcare provider if you:
- have or had liver problems. You may need a lower dose of Votrient or your healthcare provider may prescribe a different medicine to treat your advanced renal cell cancer or advanced soft tissue sarcoma.
- have high blood pressure
- have heart problems or an irregular heartbeat including QT prolongation
- have a history of a stroke
- have headaches, seizures, or vision problems
- have coughed up blood in the last 6 months
- had bleeding of your stomach or intestines in the last 6 months
- have a history of a tear (perforation) in your stomach or intestine, or an abnormal connection between two parts of your gastrointestinal tract (fistula)
- have had blood clots in a vein or in the lung
- have thyroid problems
- had recent surgery (within the last 7 days) or are going to have surgery
- have any other medical conditions
- are pregnant or plan to become pregnant. Votrient can harm your unborn baby. You should not become pregnant while you are taking Votrient. You should use effective birth control during treatment with Votrient and for at least 2 weeks after your final dose of Votrient. Talk to your healthcare provider about types of birth control that may be right for you during this time.
- are a male (including one who has had a vasectomy) with a sexual partner who is pregnant, think that they may be pregnant, or who could become pregnant (including those who use other forms of birth control). You should use condoms during sexual intercourse during treatment with Votrient and for at least 2 weeks after the last dose of Votrient.
- are breastfeeding or plan to breastfeed. It is not known if Votrient passes into your breast milk. Do not breastfeed during treatment with Votrient and for 2 weeks after the final dose.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements. Votrient may affect the way other medicines work and other medicines may affect how Votrient works.
Especially, tell your healthcare provider if you:
- take medicines that can affect how your liver enzymes work such as:
- certain antibiotics (used to treat infections)
- certain medicines used to treat depression
- certain medicines used to treat HIV
- medicines used to treat irregular heart beats
- take a medicine that contains simvastatin to treat high cholesterol levels
- take medicines that reduce stomach acid (e.g., esomeprazole)
- drink grapefruit juice
Ask your healthcare provider if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Votrient?
- Take Votrient exactly as your healthcare provider tells you. Your healthcare provider will tell you how much Votrient to take.
- Your healthcare provider may change your dose.
- Take Votrient on an empty stomach, at least 1 hour before or 2 hours after food.
- Do not crush Votrient tablets.
- Do not eat grapefruit or drink grapefruit juice during treatment with Votrient. Grapefruit products may increase the amount of Votrient in your body.
- If you miss a dose, take it as soon as you remember. Do not take it if it is close (within 12 hours) to your next dose. Just take the next dose at your regular time. Do not take more than 1 dose of Votrient at a time.
- Your healthcare provider will test your urine, blood, and heart before you start and while you take Votrient.
- Tell your healthcare provider if you plan to have surgery while taking Votrient. You will need to stop taking Votrient at least 7 days before surgery because Votrient may affect healing after surgery.
What are the possible side effects of Votrient?
- See “What is the most important information I should know about Votrient?”
- irregular or fast heartbeat or fainting
- heart failure. This is a condition where your heart does not pump as well as it should and may cause you to have shortness of breath.
- heart attack or stroke. Heart attack and stroke can happen with Votrient and may cause death.
Symptoms may include: chest pain or pressure, pain in your arms, back, neck or jaw, shortness of breath, numbness or weakness on one side of your body, trouble talking, headache, or dizziness. - blood clots. Blood clots may form in a vein, especially in your legs (deep vein thrombosis or DVT). Pieces of a blood clot may travel to your lungs (pulmonary embolism). This may be life-threatening and cause death.
Symptoms may include: new chest pain, trouble breathing or shortness of breath that starts suddenly, leg pain, and swelling of the arms and hands, or legs and feet, a cool or pale arm or leg. - thrombotic microangiopathy (TMA) including thrombotic thrombocytopenia purpura (TTP) and hemolytic uremic syndrome (HUS). TMA is a condition involving blood clots that can happen while taking Votrient. TMA is accompanied by a decrease in red blood cells and cells that are involved in clotting. TMA may harm organs such as the brain and kidneys.
- tear in your stomach or intestinal wall (perforation) or an abnormal connection between two parts of your gastrointestinal tract (fistula).
Symptoms may include: pain, swelling in your stomach area, vomiting blood, and black sticky stools. - lung problems. Votrient may cause lung problems that may lead to death. Tell your healthcare provider right away if you get a cough that will not go away or shortness of breath.
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS). RPLS is a condition that can happen while taking Votrient that may cause death.
Symptoms may include: headaches, seizures, lack of energy, confusion, high blood pressure, loss of speech, blindness or changes in vision, and problems thinking. - high blood pressure. High blood pressure can happen with Votrient, including a sudden and severe rise in blood pressure which may be life-threatening. These blood pressure increases usually happen in the first several months of treatment. Your blood pressure should be well controlled before you start taking Votrient. Your healthcare provider should begin checking your blood pressure within 1 week of you starting Votrient and often during treatment to make sure that your blood pressure is well controlled.
Have someone call your healthcare provider or get medical help right away for you, if you get symptoms of a severe increase in blood pressure, including: severe chest pain, severe headache, blurred vision, confusion, nausea and vomiting, severe anxiety, shortness of breath, seizures, or you pass out (become unconscious). - thyroid problems. Your healthcare provider should check you for this during treatment with Votrient.
- Tumor lysis syndrome (TLS). TLS is a condition that can happen during treatment with Votrient that may cause death. TLS is caused by a fast breakdown of cancer cells. Your healthcare provider may do a blood test to check you for TLS. Call your healthcare provider or get emergency medical help right away if you develop any of these symptoms during treatment with Votrient: irregular heartbeat, seizures, confusion, muscle cramps or spasms, or a decrease in urine output.
- protein in your urine. Your healthcare provider will check you for this problem. If there is too much protein in your urine, your healthcare provider may tell you to stop taking Votrient.
- serious infections. Serious infections can happen with Votrient and can cause death.
Symptoms of an infection may include: fever, cold symptoms, such as runny nose or sore throat that do not go away, flu symptoms, such as cough, tiredness, and body aches, pain when urinating, cuts, scrapes or wounds that are red, warm, swollen or painful. - collapsed lung (pneumothorax). A collapsed lung can happen with Votrient. Air may get trapped in the space between your lung and chest wall. This may cause you to have shortness of breath.
Call your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects in people who take Votrient include:
- diarrhea
- nausea or vomiting
- change in hair color
- loss of appetite
Other common side effects in people with advanced soft tissue sarcoma who take Votrient include:
- feeling tired
- headache
- decreased weight
- taste changes
- tumor pain
- trouble breathing
- muscle or bone pain
- change in skin color
- stomach pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Votrient. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Votrient
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Votrient for a condition for which it was not prescribed. Do not give Votrient to other people even if they have the same symptoms that you have. It may harm them.
If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Votrient that is written for healthcare professionals. For more information, go to www.VOTRIENT.com or call 1-888-669-6682.
How should I store Votrient?
Store Votrient at room temperature between 68°F and 77°F (20°C to 25°C).
Keep Votrient and all medicines out of the reach of children.
What are the ingredients in Votrient?
Active ingredient: pazopanib.
Inactive ingredients: Tablet core: Magnesium stearate, microcrystalline cellulose, povidone, and sodium starch glycolate. Coating: Gray film-coat: Hypromellose, iron oxide black, macrogol/polyethylene glycol 400 (PEG 400), polysorbate 80, and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-1077-66
- Votrient®
- (pazopanib)
- Tablets
- 200 mg
- 120 Tablets
- Rx only
- Each tablet contains 216.7 mg of
pazopanib hydrochloride, equivalent
to 200 mg of pazopanib free base. - Dispense with Medication Guide
attached or provided separately. - New Tablet Color
- NOVARTIS

SRC: NLM .
