Vitrakvi
Generic name: larotrectinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Vitrakvi?
Vitrakvi is a prescription medicine that is used to treat adults and children with solid tumors (cancer) that are caused by certain abnormal NTRK genes and have spread or if surgery to remove their cancer is likely to cause severe complications, and there is no acceptable treatment option or the cancer grew or spread on other treatment.
Your healthcare provider will perform a test to make sure that Vitrakvi is right for you.
It is not known if Vitrakvi is safe and effective in children younger than 1 month of age.
Description
Larotrectinib is a kinase inhibitor. VITRAKVI (larotrectinib) capsules and oral solution are formulated using larotrectinib sulfate. The molecular formula for larotrectinib sulfate is C21H24F2N6O6S and the molecular weight is 526.51 g/mol for the sulfate salt and 428.44 g/mol for the free base. The chemical name is (3S)-N-{5-[(2R)-2-(2,5-difluorophenyl)-1-pyrrolidinyl]pyrazolo[1,5-a]pyrimidin-3-yl}-3-hydroxy-1-pyrrolidinecarboxamide sulfate. Larotrectinib sulfate has the following chemical structure:
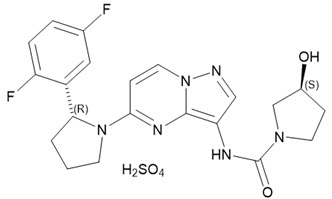
Larotrectinib sulfate is an off-white to pinkish yellow solid that is not hygroscopic. The aqueous solubility of larotrectinib at 37°C is pH dependent (very soluble at pH 1.0 and freely soluble at pH 6.8, according to USP descriptive terms of solubility).
VITRAKVI (larotrectinib) capsules and oral solution are for oral use. Each capsule contains 25 mg or 100 mg larotrectinib (30.7 mg and 123 mg larotrectinib sulfate, respectively) in a hard gelatin capsule. The capsule is composed of gelatin, titanium dioxide, and edible ink.
The oral solution contains 20 mg/mL larotrectinib (24.6 mg/mL larotrectinib sulfate) and the following inactive ingredients: purified water, hydroxypropyl betadex, sucrose, glycerin, sorbitol, citric acid, sodium phosphate, sodium citrate dihydrate, propylene glycol and flavoring. Preserved with methylparaben and potassium sorbate.
Mechanism of Action
Larotrectinib is an inhibitor of the tropomyosin receptor kinases (TRK), TRKA, TRKB, and TRKC. In a broad panel of purified enzyme assays, larotrectinib inhibited TRKA, TRKB, and TRKC with IC50 values between 5-11 nM. One other kinase TNK2 was inhibited at approximately 100-fold higher concentration. TRKA, B, and C are encoded by the genes NTRK1, NTRK2, and NTRK3. Chromosomal rearrangements involving in-frame fusions of these genes with various partners can result in constitutively-activated chimeric TRK fusion proteins that can act as an oncogenic driver, promoting cell proliferation and survival in tumor cell lines.
In in vitro and in vivo tumor models, larotrectinib demonstrated anti-tumor activity in cells with constitutive activation of TRK proteins resulting from gene fusions, deletion of a protein regulatory domain, or in cells with TRK protein overexpression. Larotrectinib had minimal activity in cell lines with point mutations in the TRKA kinase domain, including the clinically identified acquired resistance mutation, G595R. Point mutations in the TRKC kinase domain with clinically identified acquired resistance to larotrectinib include G623R, G696A, and F617L.
What should I tell my healthcare provider before taking Vitrakvi?
Before taking Vitrakvi, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems
- have nervous system (neurological) problems
- are pregnant or plan to become pregnant. Vitrakvi can harm your unborn baby. You should not become pregnant during treatment with Vitrakvi.
- If you are able to become pregnant, your healthcare provider may do a pregnancy test before you start treatment with Vitrakvi.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment and for at least 1 week after the final dose of Vitrakvi. Talk to your healthcare provider about birth control methods that may be right for you.
- Males with female partners who are able to become pregnant should use effective birth control during treatment with Vitrakvi and for at least 1 week after the final dose of Vitrakvi.
- are breastfeeding or plan to breastfeed. It is not known if Vitrakvi passes into your breast milk. Do not breastfeed during treatment and for 1 week after the last dose of Vitrakvi.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain other medicines may affect how Vitrakvi works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Vitrakvi?
- Take Vitrakvi exactly as your healthcare provider tells you.
- Your healthcare provider may stop treatment or change your dose of Vitrakvi if you have side effects. Do not change your dose or stop taking Vitrakvi unless your healthcare provider tells you.
- Vitrakvi comes in capsules and as an oral solution.
- If your healthcare provider prescribes Vitrakvi oral solution:
- Your healthcare provider will provide you with the Vitrakvi oral solution and oral syringes or send you to a pharmacy that can provide you with Vitrakvi oral solution and oral syringes.
- Your healthcare provider should show you how to correctly measure and give a dose of Vitrakvi oral solution.
- See the detailed Instructions for Use that comes with Vitrakvi oral solution for information about the correct way to measure and give a dose of Vitrakvi oral solution. If you have any questions, talk to your healthcare provider or pharmacist.
- Vitrakvi is usually taken by mouth 2 times a day.
- Swallow Vitrakvi capsules whole. Do not chew or crush the capsules.
- Take Vitrakvi with or without food.
- If you vomit after taking a dose of Vitrakvi, wait and take the next dose at your scheduled time
- If you miss a dose of Vitrakvi, take it as soon as you remember unless your next scheduled dose is due within 6 hours. Take the next dose at your regular time.
If you take too much Vitrakvi, call your healthcare provider.
What should I avoid while taking Vitrakvi?
- Vitrakvi can make you feel dizzy. Do not drive or operate machinery until you know how Vitrakvi affects you.
- Avoid taking St. John’s wort, eating grapefruit, or drinking grapefruit juice during treatment with Vitrakvi.
What are the possible side effects of Vitrakvi?
Vitrakvi may cause serious side effects, including:
- Nervous system problems. Tell your healthcare provider if you develop any symptoms such as confusion, difficulty speaking, dizziness, coordination problems, tingling, numbness, or burning sensation in your hands and feet. Your healthcare provider may temporarily stop treatment, decrease your dose, or permanently stop Vitrakvi if you develop symptoms of a nervous system problem with Vitrakvi.
- Liver problems. Your healthcare provider will do blood tests to check your liver function during treatment with Vitrakvi. Tell your healthcare provider right away if you develop symptoms of liver problems including: loss of appetite, nausea or vomiting, or pain on the upper right side of your stomach area. Your healthcare provider may temporarily stop treatment, decrease your dose, or permanently stop Vitrakvi if you develop liver problems with Vitrakvi.
The most common side effects of Vitrakvi include:
- tiredness
- nausea
- dizziness
- vomiting
- cough
- constipation
- diarrhea
Vitrakvi may affect fertility in females and may affect your ability to become pregnant. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects with Vitrakvi. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Vitrakvi
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Vitrakvi for a condition for which it was not prescribed. Do not give Vitrakvi to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for more information about Vitrakvi that is written for health professionals.
How should I store Vitrakvi?
- Store Vitrakvi capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Vitrakvi oral solution in the refrigerator between 36° F to 46° F (2° C to 8° C). Do not freeze.
- Throw away (dispose of) any unused Vitrakvi oral solution remaining after 90 days of first opening the bottle.
Keep Vitrakvi and all medicines out of the reach of children.
What are the ingredients in Vitrakvi?
Active ingredient: larotrectinib
Inactive ingredients:
Capsule: gelatin, titanium dioxide and edible ink
Oral Solution: purified water, hydroxypropyl betadex, sucrose, glycerin, sorbitol, citric acid, sodium phosphate, sodium citrate dihydrate, propylene glycol and flavoring. Preserved with methylparaben and potassium sorbate.
Label
Package Label – 25 mg – 60 Capsules
- NDC 71777-390-01
- VITRAKVI®
- (larotrectinib) capsules
- 25 mg
- Usual Dosage: See prescribing information. Rx only.
60 capsules. Keep out of reach of children.
Store at 20°C to 25°C (68°F to 77°F).
Excursions permitted from 15°C to 30°C (59° to 86°F). - Manufactured for Loxo Omcology, Inc. Stamford, CT 06901 86579626
- (01)10371777390012
- Bayer
- LOXO
- Each capsule contains
25 mg larotrectinib
(equivalent to 30.7 mg
larotrectinib sulfate).

Package Label – Carton – 100 mg – 60 Capsules
- VITRAKVI®
- (larotrectinib) capsules
- 100 mg
- Each capsule contains 100 mg larotrectinib
(equivalent to 123 mg larotrectinib sulfate). - -60 capsules
-oral use

SRC: NLM .
