Vemlidy
Generic name: tenofovir alafenamide
Drug class: Nucleoside reverse transcriptase inhibitors (NRTIs)
Medically reviewed by A Ras MD.
What is Vemlidy?
Vemlidy is a prescription medicine used to treat chronic (long-lasting) hepatitis B virus (HBV) in adults with stable (compensated) liver disease.
Vemlidy may lower the amount of HBV in your body. Vemlidy may improve the condition of your liver.
It is not known if Vemlidy is safe and effective in children under 18 years of age.
Description
VEMLIDY is a tablet containing tenofovir alafenamide for oral administration. Tenofovir alafenamide, a hepatitis B virus (HBV) nucleoside analog reverse transcriptase inhibitor, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
Each tablet contains 25 mg of tenofovir alafenamide (equivalent to 28 mg of tenofovir alafenamide fumarate). The tablets include the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film coated with a coating material containing: iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1).
It has an empirical formula of C21H29O5N6P∙½(C4H4O4) and a formula weight of 534.50. It has the following structural formula:
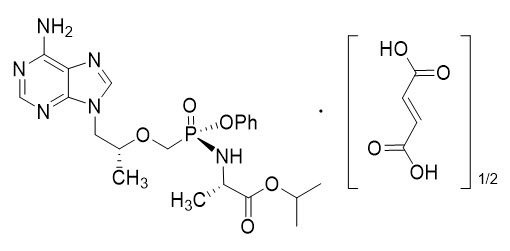
Tenofovir alafenamide fumarate is a white to off-white or tan powder with a solubility of 4.7 mg per mL in water at 20 °C.
What is the most important information I should know about Vemlidy?
Vemlidy can cause serious side effects, including:
- Worsening of hepatitis B infection. Your hepatitis B (HBV) infection may become worse (flare-up) if you take Vemlidy and then stop taking it. A “flare-up” is when your HBV infection suddenly returns in a worse way than before.
- Do not run out of Vemlidy. Refill your prescription or talk to your healthcare provider before your Vemlidy is all gone.
- Do not stop taking Vemlidy without first talking to your healthcare provider.
- If you stop taking Vemlidy, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking Vemlidy.
For more information about side effects, see the section “What are the possible side effects of Vemlidy?”
What should I tell my healthcare provider before taking Vemlidy?
Before you take Vemlidy, tell your healthcare provider about all of your medical conditions, including if you:
- have HIV-1 infection. Your healthcare provider may test you for HIV-1 infection before you start Vemlidy. If you have both HBV and HIV-1, and you only take Vemlidy, the HIV-1 virus may develop resistance and become harder to treat.
- have end stage renal disease (ESRD).
- are pregnant or plan to become pregnant. It is not known if Vemlidy will harm your unborn baby. Tell your healthcare provider if you become pregnant during treatment with Vemlidy.
Pregnancy Registry: There is a pregnancy registry for women who take antiviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk with your healthcare provider about how you can take part in this registry. - are breastfeeding or plan to breastfeed. It is not known if Vemlidy passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may affect how Vemlidy works.
- Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. You can ask your healthcare provider or pharmacist for a list of medicines that interact with Vemlidy.
- Do not start a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Vemlidy with other medicines.
How should I take Vemlidy?
- Take Vemlidy exactly as your healthcare provider tells you to take it.
- Take Vemlidy 1 time each day.
- Take Vemlidy with food.
- If you are on dialysis, on your dialysis days, take your daily dose of Vemlidy following dialysis.
- Do not change your dose or stop taking Vemlidy without first talking with your healthcare provider. Stay under a healthcare provider’s care when taking Vemlidy.
- Do not miss a dose of Vemlidy.
- If you take too much Vemlidy, call your healthcare provider or go to the nearest hospital emergency room right away.
- When your Vemlidy supply starts to run low, get more from your healthcare provider or pharmacy. This is very important because your HBV infection may get worse (flare-up) if you stop taking Vemlidy.
What are the possible side effects of Vemlidy?
Vemlidy may cause serious side effects, including:
- See “What is the most important information I should know about Vemlidy?”
- New or worse kidney problems, including kidney failure. Your healthcare provider should do blood and urine tests to check your kidneys when starting and during treatment with Vemlidy. Your healthcare provider may tell you to stop taking Vemlidy if you develop new or worse kidney problems.
- Too much lactic acid in your blood (lactic acidosis). Too much lactic acid is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms: weakness or being more tired than usual, unusual muscle pain, being short of breath or fast breathing, stomach pain with nausea and vomiting, cold or blue hands and feet, feel dizzy or lightheaded, or a fast or abnormal heartbeat.
- Severe liver problems. In rare cases, severe liver problems can happen that can lead to death. Tell your healthcare provider right away if you get these symptoms: skin or the white part of your eyes turns yellow, dark “tea-colored” urine, light-colored stools, loss of appetite for several days or longer, nausea, or stomach-area pain.
The most common side effect of Vemlidy is headache. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Vemlidy. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Vemlidy
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Vemlidy for a condition for which it was not prescribed. Do not give Vemlidy to other people, even if they have the same symptoms you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Vemlidy that is written for health professionals.
How should I store Vemlidy?
- Store Vemlidy below 86 °F (30 °C).
- Keep Vemlidy in its original container.
- Keep the container tightly closed.
- Vemlidy comes in a child-resistant package.
Keep Vemlidy and all medicines out of reach of children.
What are the ingredients in Vemlidy?
Active ingredients: tenofovir alafenamide
Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing: iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 25 mg Tablet Bottle Label
- NDC 61958- 2301-130 tablets
- Vemlidy®(tenofovir alafenamide)
tablets, 25 mg
- Note to pharmacist:Do not cover ALERT box with pharmacy label.
- ALERT: Find out about medicines thatshould NOT be taken with Vemlidy