Varubi
Generic name: rolapitant (oral – tablets)
Drug class: NK1 receptor antagonists
Medically reviewed by A Ras MD.
What is Varubi?
Varubi is a prescription medicine called an “antiemetic.” Varubi is used with other medicines in adults to help prevent nausea and vomiting that happens later with certain anti-cancer medicines (chemotherapy). It is not known if Varubi is safe and effective in children.
Description
VARUBI contains rolapitant, a substance P/neurokinin 1 (NK1) receptor antagonist. Rolapitant hydrochloride is chemically described as (5S,8S)-8- { [(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]}-8-phenyl-1,7-diazaspiro[4.5]decan-2-one hydrochloride. Its empirical formula is C25H26F6N2O2. HCl.H2O, and its structural formula is:
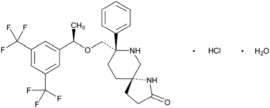 |
| rolapitant hydrochloride |
Rolapitant hydrochloride is a white to off-white powder, with a molecular weight of 554.95. Solubility of rolapitant hydrochloride in aqueous solution is pH-dependent and is more soluble at lower pH. Rolapitant has good solubility in common pharmaceutical solvents such as ethanol, propylene glycol and 40% hydroxypropyl beta-cyclodextrin.
Each tablet contains 90 mg rolapitant (equivalent to 100 mg rolapitant hydrochloride) and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch. The tablets are coated in non-functional blue and clear coats. The tablet coating comprises the following inactive ingredients: FD&C Blue No. 2-Indigo Carmine Lake, polyethylene glycol, polysorbate 80, polyvinyl alcohol, talc, and titanium dioxide.
Mechanism of Action
Rolapitant is a selective and competitive antagonist of human substance P/NK1 receptors. Rolapitant does not have significant affinity for the NK2 or NK3 receptors or for a battery of other receptors, transporters, enzymes and ion channels. Rolapitant is also active in animal models of chemotherapy-induced emesis.
Who should not take Varubi?
Do not take Varubi if you:
- take thioridazine or pimozide. Taking Varubi with thioridazine or pimozide can cause serious or life-threatening heart rhythm changes.
- are a child less than 2 years of age.
What should I tell my healthcare provider before taking Varubi?
Before taking Varubi, tell your doctor about all of your medical conditions, including if you:
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if Varubi will harm your unborn baby. Varubi may affect the ability to have a child (fertility problems) in females.
- are breastfeeding or plan to breastfeed. It is not known if Varubi passes into your breast milk or could harm your baby. Talk to your doctor about the best way to feed your baby if you take Varubi.
Tell your doctor about all the medicines you take or stop taking, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Keep a list of your medicines to show your doctor and pharmacist when you get a new medicine.
Varubi and other medicines may affect each other and could cause serious side effects.
How should I take Varubi?
- Take Varubi exactly as your doctor tells you to take it.
- Varubi is taken on Day 1 of chemotherapy. Take 2 Varubi tablets by mouth within 2 hours before you receive your anti-cancer medicine (chemotherapy).
- Varubi can be taken with or without food.
- Do not take Varubi more than 1 time every 14 days.
- If you take too much Varubi, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of Varubi?
Varubi may cause serious side effects, including:
- Change in the level of some medicines in your blood. Serious or life-threatening reactions, including heart rhythm changes, may occur if Varubi is used with certain other medicines. You should not take Varubi if you take thioridazine or pimozide.
The most common side effects of Varubi in people who take Varubi and receive Cisplatin chemotherapy medicine include: low white blood cell count, hiccups, nausea, and stomach (abdominal) pain.
The most common side effects of Varubi in people who take Varubi and receive Anthracycline and Cyclophosphamide chemotherapy medicines include: decreased appetite, low white blood cell count, dizziness, indigestion, urinary tract infection, mouth sores, and low red blood cell count.
These are not all the possible side effects of Varubi. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Varubi
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Varubi for a condition for which it was not prescribed. Do not give Varubi to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about Varubi that is written for health professionals.
How should I store Varubi?
- Varubi tablets come in a child-resistant package.
- Store Varubi at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Varubi and all medicines out of the reach of children.
What are the ingredients in Varubi?
Active ingredient: rolapitant
Inactive ingredients (tablet): colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and pregelatinized starch. The tablets are coated in non-functional blue and clear coats. The tablet coating comprises the following inactive ingredients: FD&C Blue No. 2-Indigo Carmine Lake, polyethylene glycol, polysorbate 80, polyvinyl alcohol, talc, and titanium dioxide.
Label
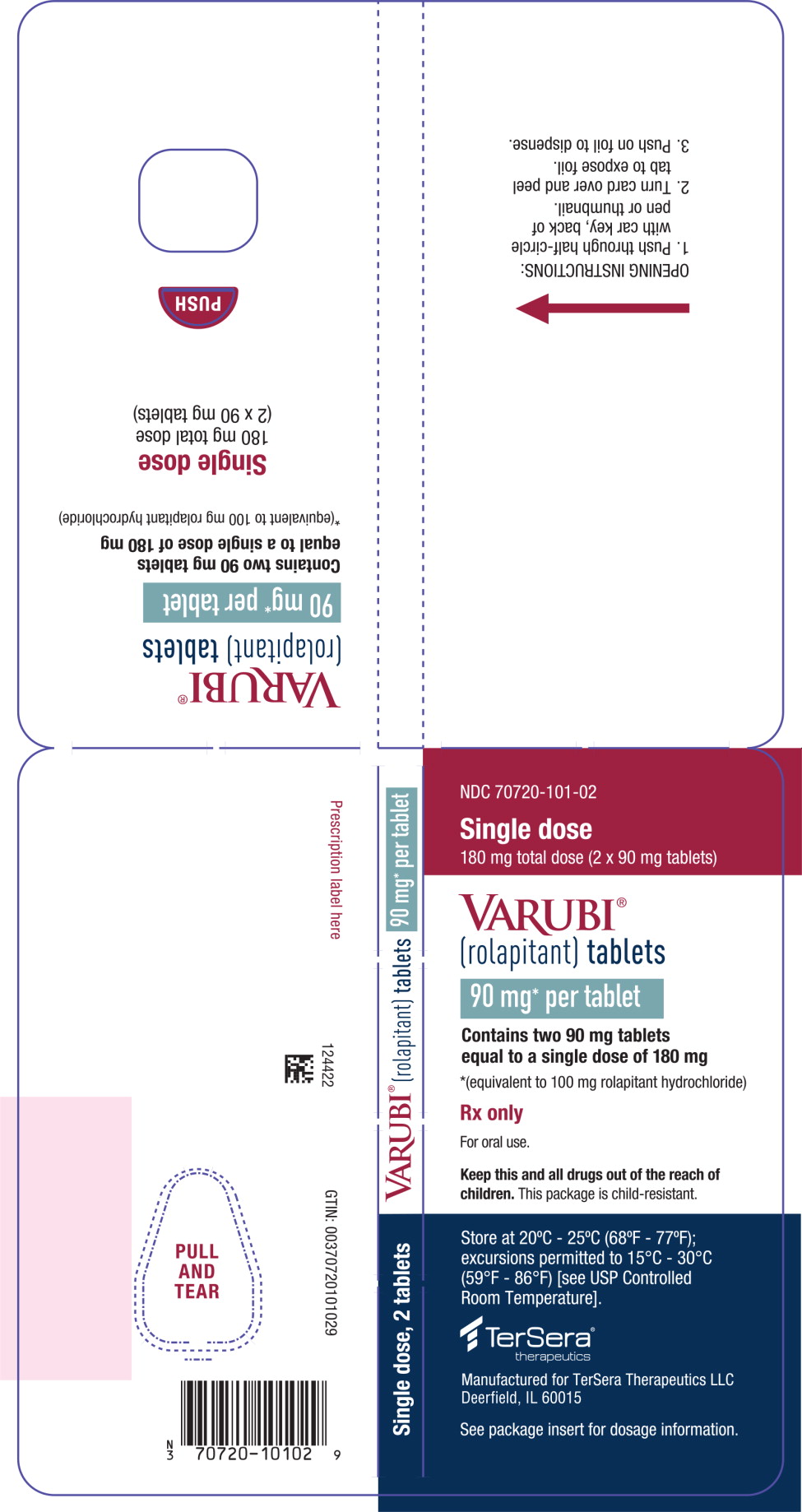
PRINCIPAL DISPLAY PANEL – 90 mg Tablet Blister Wallet
- NDC 70720-101-02
- Single dose
180 mg total dose (2 x 90 mg tablets) - VARUBI®
(rolapitant) tablets - 90 mg* per tablet
- Contains two 90 mg tablets
equal to a single dose of 180 mg - *(equivalent to 100 mg rolapitant hydrochloride)
- Rx only
- For oral use.
- Keep this and all drugs out of the reach of
children. This package is child-resistant. - Store at 20°C – 25°C (68°F – 77°F);
excursions permitted to 15°C – 30°C
(59°F – 86°F) [see USP Controlled
Room Temperature]. - TerSera®
therapeutics - Manufactured for TerSera Therapeutics LLC
Deerfield, IL 60015 - See package insert for dosage information.
SRC: NLM .
