Ultracet
Generic name: acetaminophen and tramadol
Drug class: Narcotic analgesic combinations
Medically reviewed by A Ras MD.
What is Ultracet?
Ultracet is a strong prescription pain medicine that contains an opioid (narcotic) that is used for the short-term (five days or less) management of acute pain, when other pain treatments such as non-opioid pain medicines do not treat your pain well enough or you cannot tolerate them. It is an opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death.
Description
ULTRACET® (tramadol hydrochloride/acetaminophen) Tablets combines two analgesics, tramadol hydrochloride and opioid agonist, and acetaminophen.
The chemical name for tramadol hydrochloride is (±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:

The molecular weight of tramadol hydrochloride is 299.84. Tramadol hydrochloride is a white, bitter, crystalline, and odorless powder.
The chemical name for acetaminophen is N-acetyl-p-aminophenol. Its structural formula is:

The molecular weight of acetaminophen is 151.17. Acetaminophen is an analgesic and antipyretic agent which occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste.
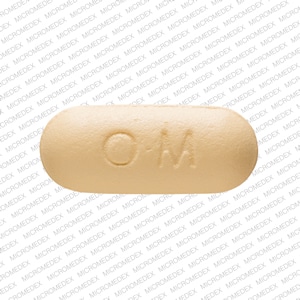
ULTRACET tablets contain 37.5 mg of tramadol hydrochloride and 325 mg acetaminophen and are light yellow in color. Inactive ingredients in the tablet are carnauba wax, corn starch, hypromellose, iron oxide, magnesium stearate, polyethylene glycol, polysorbate 80, powdered cellulose, pregelatinized corn starch, sodium starch glycolate, and titanium dioxide.
Meets USP Dissolution Test 2.
Mechanism of Action
ULTRACET contains tramadol, an opioid agonist and inhibitor of norepinephrine and serotonin re-uptake, and acetaminophen. Although the mode of action of tramadol is not completely understood, the analgesic effect of tramadol is believed to be due to both binding to µ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
Opioid activity of tramadol is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to µ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in µ-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests. The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound .
Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol.
Acetaminophen is a non-opioid, non-salicylate analgesic. The site and mechanism for the analgesic effect of acetaminophen has not been determined but is thought to primarily involve central actions.
What is the most important information I should know about Ultracet?
- Get emergency help right away if you take too much Ultracet (overdose). When you first start taking Ultracet, when your dose is changed, or if you take too much (overdose), serious or life-threatening breathing problems that can lead to death may occur.
- Ultracet can cause severe drowsiness, breathing problems (respiratory depression), coma and death when taken with benzodiazepines or other medicines that depress consciousness.
- Never give anyone else your Ultracet. They could die from taking it. Selling or giving away Ultracet is against the law.
- Store Ultracet securely, out of sight and reach of children, and in a location not accessible by others, including visitors to the home.
- Get emergency help right away if you take more than 4,000 mg of acetaminophen in 1 day. Taking Ultracet with other products that contain acetaminophen can lead to serious liver problems and death.
Important Information Guiding Use in Pediatric Patients:
- Do not give Ultracet to a child younger than 12 years of age.
- Do not give Ultracet to a child younger than 18 years of age after surgery to remove the tonsils and/or adenoids.
- Avoid giving Ultracet to children between 12 to 18 years of age who have risk factors for breathing problems such as obstructive sleep apnea, obesity, or underlying lung problems.
Who should not take Ultracet?
Do not take Ultracet if you have:
- Severe asthma, trouble breathing, or other lung problems.
- A bowel blockage or have narrowing of the stomach or intestines.
- An allergy to any of its ingredients (e.g., tramadol hydrochloride or acetaminophen).
- Taken a Monoamine Oxidase Inhibitor, MAOI, (medicine used for depression) within the last 14 days.
What should I tell my healthcare provider before taking Ultracet?
Before taking Ultracet, tell your healthcare provider if you have a history of:
- head injury, seizures
- problems urinating
- liver, kidney, thyroid problems
- pancreas or gallbladder problems
- abuse of street or prescription drugs, alcohol addiction, or mental health problems.
Tell your healthcare provider if you are:
- pregnant or planning to become pregnant. Prolonged use of Ultracet during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- breastfeeding. Not recommended; it may harm your baby.
- taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking Ultracet with certain other medicines can cause serious side effects that could lead to death.
How should I take Ultracet?
When taking Ultracet:
- Do not change your dose. Take Ultracet exactly as prescribed by your healthcare provider. Use the lowest dose possible for the shortest time needed.
- Take your prescribed dose: 2 tablets every 4 to 6 hours as needed for pain relief for a maximum of 5 days. Do not take more than your prescribed dose and do not take more than 8 tablets per day. If you miss a dose, take your next dose at your usual time.
- Call your healthcare provider if the dose you are taking does not control your pain.
- If you have been taking Ultracet regularly, do not stop taking Ultracet without talking to your healthcare provider.
- Dispose of expired, unwanted, or unused Ultracet by taking your drug to an authorized Drug Enforcement Administration (DEA)-registered collector or drug take-back program. If one is not available, you can dispose of Ultracet by mixing the product with dirt, cat litter, or coffee grounds; placing the mixture in a sealed plastic bag and throwing the bag in your trash.
What should I avoid while taking Ultracet?
While taking Ultracet do not:
- Drive or operate heavy machinery, until you know how Ultracet affects you. Ultracet can make you sleepy, dizzy, or lightheaded.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol. Using products containing alcohol during treatment with Ultracet may cause you to overdose and die.
What are the possible side effects of Ultracet?
The possible side effects of Ultracet:
- constipation
- nausea
- sleepiness
- vomiting
- tiredness
- headache
- dizziness
- abdominal pain
Call your healthcare provider if you have any of these symptoms and they are severe.
Get emergency medical help if you have:
- trouble breathing
- shortness of breath
- fast heartbeat
- chest pain
- swelling of your face, tongue, or throat
- extreme drowsiness
- light-headedness when changing positions
- feeling faint
- agitation
- high body temperature
- trouble walking
- stiff muscles
- mental changes such as confusion
These are not all the possible side effects of Ultracet. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Ultracet?
Store at 20 – 25°C (68 – 77°F); excursions permitted to 15 – 30°C (59 – 86°F).
Store Ultracet securely and dispose of properly.
What are the ingredients in Ultracet?
Active ingredients: tramadol hydrochloride, acetaminophen
Inactive ingredients: powdered cellulose; sodium starch glycolate type a potato; starch, corn; magnesium stearate; hypromellose, unspecified; polyethylene glycol, unspecified; polysorbate 80; titanium dioxide; ferrosoferric oxide; carnauba wax.
Label
PRINCIPAL DISPLAY PANEL – 37.5 MG/325 MG TABLET BOTTLE LABEL
- NDC 50458-650-60
- ULTRACET®
CIV
(37.5 mg tramadol
HCl/325 mg
acetaminophen
tablets) - Please see the
Medication
Guide provided
by your pharmacist. - Keep out of reach of children.
- Rx only
100 tablets

SRC: NLM .
