Tracleer
Generic name: bosentan
Drug class: Agents for pulmonary hypertension
Medically reviewed by A Ras MD.
What is Tracleer?
Tracleer is a prescription medicine used to treat people with certain types of pulmonary arterial hypertension (PAH), which is high blood pressure in the vessels of the lungs.
Tracleer can improve your ability to exercise and can slow the worsening of your physical condition and symptoms. Tracleer lowers high blood pressure in your lungs and lets your heart pump blood more efficiently.
Tracleer is only:
Prescribed by healthcare providers who are enrolled in the Bosentan REMS Program.
Available to people who understand and agree to enroll in the Bosentan REMS Program.
Description
TRACLEER® is the proprietary name for bosentan, an endothelin receptor antagonist that belongs to a class of highly substituted pyrimidine derivatives, with no chiral centers. It is designated chemically as 4-tert-butyl-N-[6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-[2,2´]-bipyrimidin-4-yl]- benzenesulfonamide monohydrate and has the following structural formula:
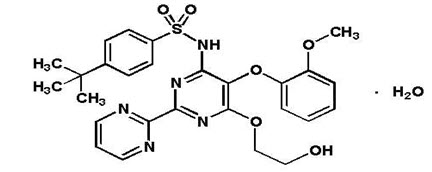
Bosentan has a molecular weight of 569.64 and a molecular formula of C27H29N5O6S∙H2O. Bosentan is a white to yellowish powder. It is poorly soluble in water (1.0 mg/100 mL) and in aqueous solutions at low pH (0.1 mg/100 mL at pH 1.1 and 4.0; 0.2 mg/100 mL at pH 5.0). Solubility increases at higher pH values (43 mg/100 mL at pH 7.5). In the solid state, bosentan is very stable, is not hygroscopic and is not light sensitive.
TRACLEER is available as 62.5 mg and 125 mg film-coated tablets for oral administration, and contains the following excipients: corn starch, ethylcellulose, glyceryl behenate, hydroxypropylmethylcellulose, iron oxide red, iron oxide yellow, magnesium stearate, povidone, pregelatinized starch, sodium starch glycolate, talc, titanium dioxide, and triacetin. Each TRACLEER 62.5 mg tablet contains 64.54 mg of bosentan monohydrate, equivalent to 62.5 mg of anhydrous bosentan. Each TRACLEER 125 mg tablet contains 129.08 mg of bosentan monohydrate, equivalent to 125 mg of anhydrous bosentan.
TRACLEER is also available as a 32 mg tablet for oral suspension and contains the following excipients: acesulfame potassium, aspartame (E951), calcium hydrogen phosphate anhydrous, cellulose microcrystalline, croscarmellose sodium, magnesium stearate, silica colloidal anhydrous, tartaric acid, and tutti frutti flavor. Each dispersible tablet contains 1.87 mg of phenylalanine. Each dispersible tablet contains 33.045 mg of bosentan monohydrate, equivalent to 32 mg anhydrous bosentan.
Mechanism of Action
Bosentan is a specific and competitive antagonist at endothelin receptor types ETA and ETB. Bosentan has a slightly higher affinity for ETA receptors than for ETB receptors. The clinical impact of dual endothelin blockage is unknown.
Endothelin-1 (ET-1) is a neurohormone, the effects of which are mediated by binding to ETA and ETB receptors in the endothelium and vascular smooth muscle. ET-1 concentrations are elevated in plasma and lung tissue of patients with PAH, suggesting a pathogenic role for ET-1 in this disease.
What is the most important information I should know about Tracleer?
Tracleer is only available through the Bosentan REMS Program. Before you begin taking Tracleer, you must read and agree to all of the instructions in the Bosentan REMS Program.
Tracleer can cause serious side effects including:
Liver damage.
- Liver damage may not cause symptoms at first. Only a blood test can show if you have early liver damage. You must have your blood tested to check your liver function before you start Tracleer and each month after that. Your healthcare provider will order these tests. Regular blood tests are important because they will help your healthcare provider adjust or stop your treatment before there is permanent damage.
- Tell your healthcare provider if you have had liver problems, including liver problems while taking other medicines. Call your healthcare provider right away if you have any of these symptoms of liver problems while taking Tracleer:
Serious birth defects.
- Tracleer can cause serious birth defects if taken during pregnancy. You must not be pregnant when you start taking Tracleer or during Tracleer treatment. Serious birth defects from Tracleer can happen early in pregnancy. Females who are able to get pregnant must have a negative pregnancy test before starting treatment with Tracleer, each month during treatment with Tracleer, and 1 month after stopping treatment with Tracleer.
- Talk to your healthcare provider about your menstrual cycle. Your healthcare provider will decide when to do a pregnancy test and will order a pregnancy test for you depending on your menstrual cycle.
- Females who are able to get pregnant are females who:
- have entered puberty, even if they have not started their menstrual period, and
- have a uterus, and
- have not gone through menopause. Menopause means that you have not had a menstrual period for at least 12 months for natural reasons, or that you have had your ovaries removed.
- Females who are not able to get pregnant are females who:
- have not yet entered puberty, or
- do not have a uterus, or
- have gone through menopause. Menopause means that you have not had a menstrual period for at least 12 months for natural reasons, or that you have had your ovaries removed or
- are infertile for other medical reasons and this infertility is permanent and cannot be reversed.
- Females who are able to get pregnant are females who:
- Talk to your healthcare provider about your menstrual cycle. Your healthcare provider will decide when to do a pregnancy test and will order a pregnancy test for you depending on your menstrual cycle.
- Females who are able to get pregnant must use two acceptable forms of birth control during treatment with Tracleer, and for one month after stopping Tracleer because the medicine may still be in the body.
- If you have had a tubal sterilization or have an IUD (intrauterine device), these methods can be used alone and no other form of birth control is needed.
- Talk with your healthcare provider or gynecologist (a doctor who specializes in female reproduction) to find out about options for acceptable birth control that you may use to prevent pregnancy during treatment with Tracleer.
- If you decide that you want to change the form of birth control that you use, talk with your healthcare provider or gynecologist to be sure that you choose another acceptable form of birth control.
See the chart below for Acceptable Birth Control Options during treatment with Tracleer.
- Do not have unprotected sex. Talk to your healthcare provider or pharmacist right away if you have unprotected sex or if you think your birth control has failed. Your healthcare provider may talk with you about using emergency birth control.
- Tell your healthcare provider right away if you miss a menstrual period or think you may be pregnant.
If you are the parent or caregiver of a female child who started taking Tracleer before reaching puberty, you should check your child regularly to see if she is developing signs of puberty. Tell your healthcare provider right away if you notice that she is developing breast buds or any pubic hair. Your healthcare provider should decide if your child has reached puberty. Your child may reach puberty before having her first menstrual period.
Acceptable birth control options
Option 1: One method from this list
- Standard intrauterine device (Copper T 380A IUD)
- Intrauterine system (LNg 20 IUS: progesterone IUS)
- Tubal sterilization
OR
Option 2: One method from this list
- Estrogen and progesterone oral contraceptives (“the pill”)
- Estrogen and progesterone transdermal patch
- Vaginal ring
- Progesterone injection
- Progesterone implant
PLUS One method from this list:
- Male condom
- Diaphragm with spermicide
- Cervical cap with spermicide
OR
Option 3: One method from this list
- Diaphragm with spermicide
- Cervical cap with spermicide
PLUS One method from this list:
- Male condom
OR
Option 4: One method from this list
- Partner’s vasectomy
PLUS One method from this list:
- Male condom
- Diaphragm with spermicide
- Cervical cap with spermicide
- Estrogen and progesterone oral contraceptives (“the pill”)
- Estrogen and progesterone transdermal patch
- Vaginal ring
- Progesterone injection
- Progesterone implant
Tracleer 32 mg dispersible tablets contain Aspartame. Phenylketonurics: Contains Phenylalanine 1.87 mg per 32 mg dispersible tablet.
See “What are the possible side effects of Tracleer?” for more information about side effects.
Who should not take Tracleer?
Do not take Tracleer if you:
- are pregnant, plan to become pregnant, or become pregnant during Tracleer treatment. Tracleer can cause serious birth defects. All females should read the birth defects section of “What is the most important information I should know about Tracleer?”
- take any of these medicines:
- cyclosporine A used to treat psoriasis and rheumatoid arthritis, and to prevent rejection of heart, liver, and kidney transplants
- glyburide used to treat diabetes
- are allergic to bosentan or any of the ingredients in Tracleer. See the end of this Medication Guide for a complete list of the ingredients in Tracleer. If you have a rash, hives or your lips swell after taking Tracleer, it may be a sign of allergy. You should stop taking your Tracleer and talk to your healthcare provider.
What should I tell my healthcare provider before taking Tracleer?
Tracleer may not be right for you. Tell your healthcare provider about all your medical conditions, including if you:
- have liver problems.
- are breast-feeding or plan to breast feed. It is not known if Tracleer passes into your milk. You and your healthcare provider should decide if you will take Tracleer or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tracleer and other medicines may affect how each other works and cause side effects. Especially tell your healthcare provider if you take:
- hormone-based birth control, such as pills, shots, patches, and implants. These birth control methods may not work as well when taken with Tracleer.
- simvastatin or other “-statin” medicines used to lower cholesterol
- rifampin used for tuberculosis
- ketoconazole, fluconazole, itraconazole, or voriconazole used for fungal infections
- warfarin sodium used to prevent blood clots
- ritonavir used to treat HIV
There may be more than one brand name medicine. Ask your healthcare provider if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show it to your healthcare provider or pharmacist when you get a new medicine.
How should I take Tracleer?
Your healthcare provider will give you detailed information about the Bosentan REMS Program.
- Tracleer is only available through certified pharmacies. You will only receive a 30-day supply of Tracleer at one time.
- Take Tracleer exactly as prescribed.
- Your healthcare provider will tell you how much Tracleer to take and when to take it.
- In most cases, you will take 1 tablet in the morning and 1 in the evening.
- You can take Tracleer orally with or without food.
- If you take more than the prescribed dose of Tracleer, call your healthcare provider right away.
- If you miss a dose of Tracleer, take your tablet as soon as you remember. Do not take 2 doses at the same time. If it is almost time for your next dose, skip the missed dose. Just take the next dose at your regular time.
- Do not stop taking Tracleer unless your healthcare provider tells you to. Suddenly stopping your treatment may cause your symptoms to get worse. If you need to stop taking Tracleer, speak with your healthcare provider about the right way to stop.
- If necessary, the 32 mg dispersible tablet can be divided into halves by breaking along the lines cut into the surface. Hold the tablet between the thumb and index finger on either side of one of the lines, with the line facing upwards, and break the tablet along the line (see figure below). The dispersible tablet should not be broken into quarters.
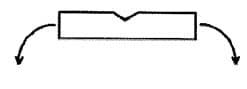
- Each 32 mg dispersible tablet, or tablet part, can be dispersed in a minimal amount of water to make a liquid medicine immediately before administration. When the tablet has fully dispersed, the liquid should be administered to the patient.
What are the possible side effects of Tracleer?
Tracleer can cause serious side effects, including:
- See “What is the most important information I should know about Tracleer?”
- Fluid retention and swelling of your ankles and legs. Tracleer can cause your body to hold too much water, and you may get swelling of your ankles and legs. Tell your healthcare provider if you have swelling of your ankles and legs that happens either with or without weight gain, or if you have more trouble with your breathing than normal. Your healthcare provider will look for the cause of this.
- Lower Sperm Count. Some men who take Tracleer may have lower sperm counts. This may affect your ability to father a child. Tell your healthcare provider if fertility is a concern for you.
- Low red blood cell levels (anemia). Your healthcare provider will do blood tests to check your red blood cells during treatment with Tracleer.
The most common side effects of Tracleer include:
- respiratory tract infection
- headache
- fainting
- flushing
- low blood pressure
- inflamed nose passages (sinusitis)
- joint pain
- irregular heart beats
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Tracleer. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tracleer
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Tracleer for a condition for which it was not prescribed. Do not give Tracleer to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Tracleer. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Tracleer that is written for health professionals.
For more information, go to www.Tracleer.com or call 1-866-228-3546.
How should I store Tracleer?
- Store Tracleer at room temperature between 68°F to 77°F (20°C to 25°C).
- Dispersible tablets that have been broken to adjust the dose of medicine can be stored at room temperature, between 68°F to 77°F (20°C to 25°C), and should be used within 7 days of having been broken. Tablet pieces may be returned to the opened blister and stored there out of reach of children for up to 7 days.
Keep Tracleer and all medicines out of the reach of children.
What are the ingredients in Tracleer?
Active ingredient: bosentan
Inactive ingredients:
62.5 mg and 125 mg film-coated tablets: corn starch, pregelatinized starch, sodium starch glycolate, povidone, glyceryl behenate, and magnesium stearate, hydroxypropylmethylcellulose, triacetin, talc, titanium dioxide, iron oxide yellow, iron oxide red, ethylcellulose.
32 mg dispersible tablets: cellulose microcrystalline, calcium hydrogen, phosphate anhydrous, croscarmellose sodium, silica colloidal anhydrous, tartaric acid, tutti frutti flavor, aspartame (E951), acesulfame potassium, and magnesium stearate.
Label
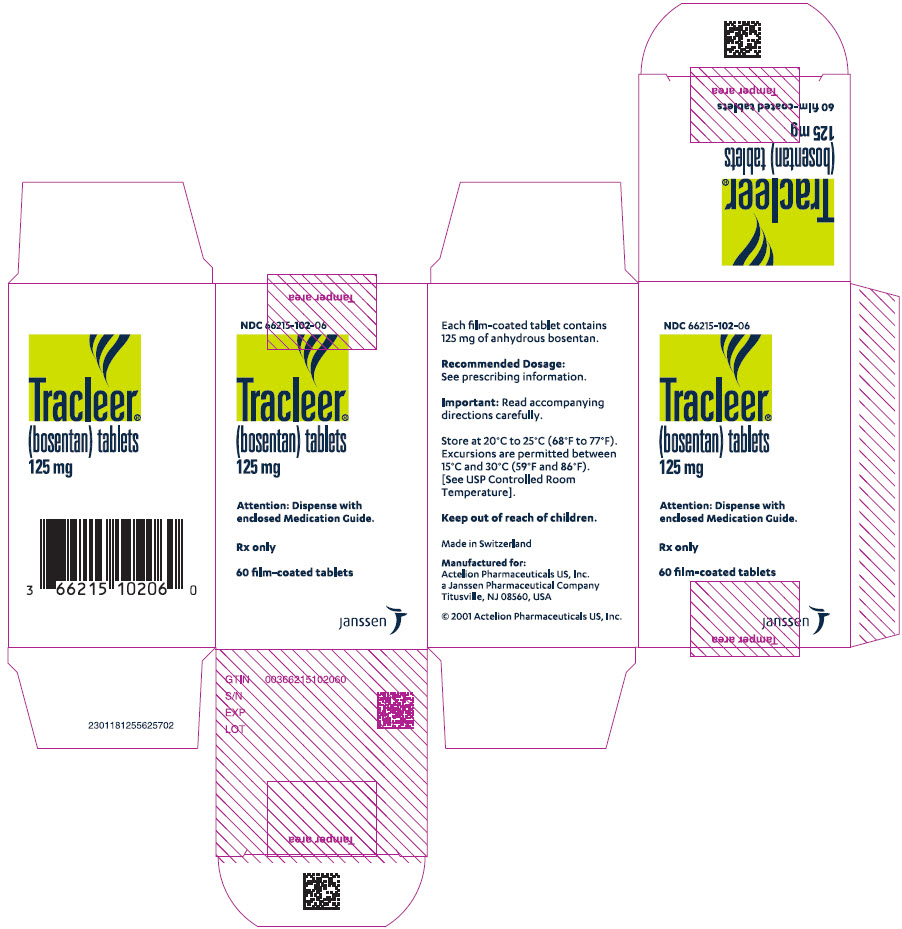
PRINCIPAL DISPLAY PANEL – 125 MG TABLET BOTTLE CARTON
- NDC 66215-102-06
- Tracleer®
(bosentan) tablets
125 mg - Attention: Dispense with
enclosed Medication Guide. - Rx only
- 60 film-coated tablets
- janssen
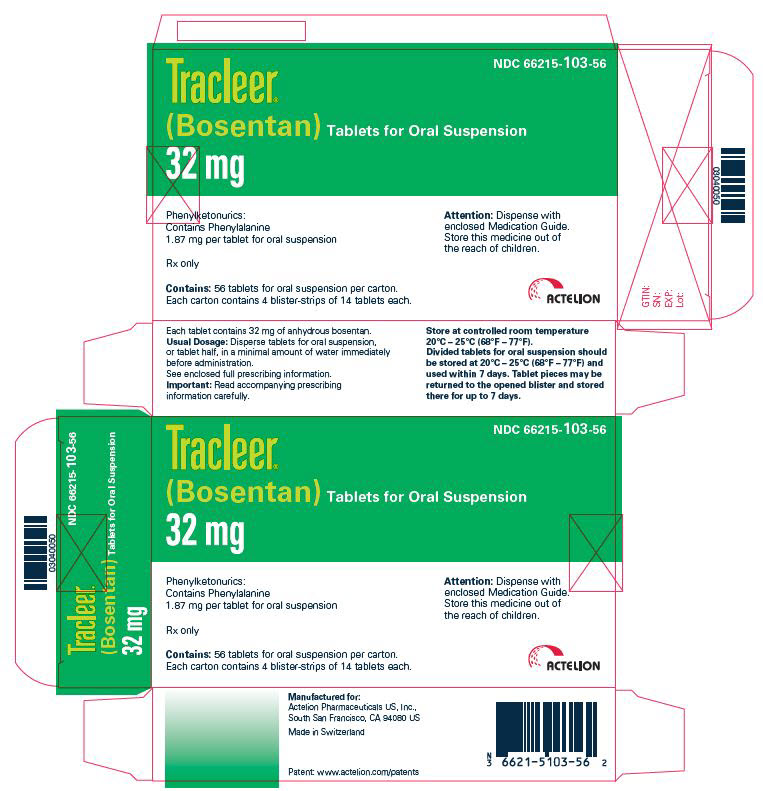
PRINCIPAL DISPLAY PANEL – 32 MG TABLET BLISTER PACK CARTON
- NDC 66215-103-56
- TRACLEER®
(Bosentan) Tablets for Oral Suspension - 32 mg
- Phenylketonurics:
Contains Phenylalanine
1.87 mg per tablet for oral suspension - Rx only
- Contains: 56 tablets for oral suspension per carton.
Each carton contains 4 blister-strips of 14 tablets each. - Attention: Dispense with
enclosed Medication Guide.
Store this medicine out of
the reach of children. - ACTELION