Tivicay
Generic name: dolutegravir
Brand names: Tivicay, Tivicay PD
Drug class: Integrase strand transfer inhibitor
Medically reviewed by A Ras MD.
What is Tivicay and Tivicay PD?
Tivicay and Tivicay PD are prescription medicines used to treat Human Immunodeficiency Virus-1 (HIV-1) infection together with:
- other HIV-1 medicines in adults who have not received HIV-1 medicines in the past or to replace their current HIV-1 medicines.
- other HIV-1 medicines in children, aged at least 4 weeks and weighing at least 6.6 pounds (3 kg), who have not received HIV-1 medicines in the past or to replace their current HIV-1 medicines when their healthcare provider determines that they meet certain requirements.
Tivicay is used together with rilpivirine as a complete regimen to treat Human Immunodeficiency Virus-1 (HIV-1) infection in adults to replace their current HIV-1 medicines when their healthcare provider determines that they meet certain requirements.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
It is not known if Tivicay or Tivicay PD is safe and effective in children who are less than 4 weeks of age and weigh less than 6.6 pounds (3 kg) or in children who have received certain types of medicine for HIV-1 infection.
Description
TIVICAY contains dolutegravir, as dolutegravir sodium, an HIV INSTI. The chemical name of dolutegravir sodium is sodium (4R,12aS)-9-{[(2,4-difluorophenyl)methyl]carbamoyl}-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1′,2′:4,5]pyrazino[2,1-b][1,3]oxazin-7-olate. The empirical formula is C20H18F2N3NaO5 and the molecular weight is 441.36 g per mol.
Structural formula:
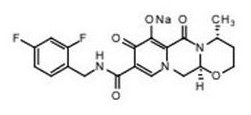
Dolutegravir sodium is a white to light yellow powder and is slightly soluble in water.
Each film-coated tablet of TIVICAY for oral administration contains 10.5, 26.3, or 52.6 mg of dolutegravir sodium, which is equivalent to 10, 25, or 50 mg dolutegravir free acid, respectively, and the following inactive ingredients: D-mannitol, microcrystalline cellulose, povidone K29/32, sodium starch glycolate, and sodium stearyl fumarate. The tablet film‑coating contains the inactive ingredients iron oxide yellow (25-mg and 50-mg tablets only), macrogol/PEG, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
Each TIVICAY PD tablet for oral suspension contains 5.26 mg of dolutegravir sodium, which is equivalent to 5 mg dolutegravir free acid, and the following inactive ingredients: calcium sulfate dihydrate, crospovidone, mannitol, microcrystalline cellulose, povidone K29/32, silicified microcrystalline cellulose, sodium starch glycolate, strawberry cream flavor, sucralose, and sodium stearyl fumarate. The tablet film-coating contains hypromellose, polyethylene glycol, and titanium dioxide.
Who should not take Tivicay and Tivicay PD?
- have ever had an allergic reaction to a medicine that contains dolutegravir.
- take dofetilide.
What should I tell my healthcare provider before taking Tivicay and Tivicay PD?
Before you take Tivicay or Tivicay PD, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had liver problems, including hepatitis B or C infection.
- are pregnant or plan to become pregnant. Tivicay or Tivicay PD may harm your unborn baby.
- Your healthcare provider may prescribe a different medicine than Tivicay or Tivicay PD if you are planning to become pregnant or if pregnancy is confirmed during the first 12 weeks of pregnancy
- If you can become pregnant, your healthcare provider will perform a pregnancy test before you start treatment with Tivicay or Tivicay PD.
- If you can become pregnant, you should consistently use effective birth control (contraception) during treatment with Tivicay or Tivicay PD.
- Tell your healthcare provider right away if you are planning to become pregnant, you become pregnant, or think you may be pregnant during treatment with Tivicay or Tivicay PD.
Pregnancy Registry. There is a pregnancy registry for individuals who take antiretroviral medicines, including Tivicay and Tivicay PD, during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk with your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Tivicay or Tivicay PD.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if Tivicay or Tivicay PD can pass to your baby in your breast milk.
Talk with your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines interact with Tivicay or Tivicay PD. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Tivicay or Tivicay PD.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Tivicay or Tivicay PD with other medicines.
How should I take Tivicay and Tivicay PD?
- Take Tivicay or Tivicay PD exactly as your healthcare provider tells you to take it.
- Take Tivicay or Tivicay PD with or without food.
- For children who cannot swallow tablets, read the Instructions for Use that come with Tivicay PD for detailed instructions on how to prepare a dose of Tivicay PD tablets for oral suspension.
- Tivicay PD may be swallowed whole or dispersed in drinking water and should not be chewed, cut, or crushed.
- Tivicay tablets are not the same as Tivicay PD tablets for oral suspension and cannot be substituted for each other. Check to make sure you receive the correct form of Tivicay each time you or your child’s prescription is filled to avoid using the wrong medicine.
- Do not change your dose, switch medicines or stop taking Tivicay or Tivicay PD without talking with your healthcare provider first.
- If you take antacids, laxatives, or other medicines that contain aluminum, magnesium, or buffered medicines, Tivicay or Tivicay PD should be taken at least 2 hours before or 6 hours after you take these medicines.
- If you need to take iron or calcium supplements by mouth during treatment with Tivicay or Tivicay PD:
- If you take Tivicay with food, you may take these supplements at the same time that you take Tivicay.
- If you do not take Tivicay or Tivicay PD with food, take Tivicay or Tivicay PD at least 2 hours before or 6 hours after you take these supplements.
- Do not miss a dose of Tivicay or Tivicay PD.
- If you miss a dose of Tivicay or Tivicay PD, take it as soon as you remember. Do not take 2 doses at the same time or take more than your prescribed dose.
- Stay under the care of a healthcare provider during treatment with Tivicay or Tivicay PD.
- Do not run out of Tivicay or Tivicay PD. The virus in your blood may increase and the virus may become harder to treat. When your supply starts to run low, get more from your healthcare provider or pharmacy.
- If you take too much Tivicay or Tivicay PD, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Tivicay and Tivicay PD?
Tivicay or Tivicay PD can cause serious side effects including:
- Allergic reactions. Call your healthcare provider right away if you develop a rash with Tivicay or Tivicay PD. Stop taking Tivicay or Tivicay PD and get medical help right away if you develop a rash with any of the following signs or symptoms:
- fever
- generally ill feeling
- tiredness
- muscle or joint aches
- blisters or sores in mouth
- blisters or peeling of the skin
- redness or swelling of the eyes
- swelling of the mouth, face, lips, or tongue
- problems breathing
- Liver problems. People with a history of hepatitis B or C virus may have an increased risk of developing new or worsening changes in certain liver tests during treatment with Tivicay or Tivicay PD. Liver problems, including liver failure, have also happened in people without a history of liver disease or other risk factors. Your healthcare provider may do blood tests to check your liver. Call your healthcare provider right away if you develop any of the following signs or symptoms of liver problems:
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after you start taking Tivicay or Tivicay PD.
The most common side effects of Tivicay include:
- trouble sleeping
- tiredness
- headache
These are not all the possible side effects of Tivicay or Tivicay PD. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of Tivicay and Tivicay PD
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Tivicay or Tivicay PD for a condition for which it was not prescribed. Do not give Tivicay or Tivicay PD to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Tivicay that is written for health professionals. For more information, go to www.Tivicay.com or call 1-877-844-8872.
How should I store Tivicay and Tivicay PD?
- Store Tivicay 10-mg, 25-mg, and 50-mg tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Tivicay 10-mg tablets in the original bottle. Keep the bottle tightly closed and protected from moisture. The bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Do not remove the desiccant packet from the bottle.
- Store Tivicay PD 5-mg tablets for oral suspension at room temperature below 86°F (30°C) in the original bottle. Keep the bottle tightly closed and protected from moisture. The bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Do not remove the desiccant packet from the bottle.
Keep Tivicay, Tivicay PD, and all medicines out of the reach of children.
What are the ingredients in Tivicay and Tivicay PD?
Active ingredient: dolutegravir.
Inactive ingredients:
Tivicay tablets: D-mannitol, microcrystalline cellulose, povidone K29/32, sodium starch glycolate, and sodium stearyl fumarate. The tablet film‑coating contains the inactive ingredients iron oxide yellow (for the 25-mg and 50-mg tablets only), macrogol/PEG, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
Tivicay PD tablets for oral suspension: calcium sulfate dihydrate, crospovidone, mannitol, microcrystalline cellulose, povidone K29/32, silicified microcrystalline cellulose, sodium starch glycolate, strawberry cream flavor, sucralose, and sodium stearyl fumarate,. The tablet film-coating contains hypromellose, polyethylene glycol, and titanium dioxide.
Instructions for use for Tivicay PD
Tivicay PD (TIV-eh-kay Pe De)
(dolutegravir) tablets for oral suspension
5 mg
Read this Instructions for Use before giving a dose of medicine.
Follow the steps below, using clean drinking water to prepare and give a dose to an infant or a child who cannot swallow the tablets.
Important information
- Always give this medicine exactly as your healthcare provider tells you. Talk to your healthcare provider if you are not sure.
- Do not chew, cut, or crush the tablets.
- If you forget to give a dose of medicine, give it as soon as you remember. Do not give 2 doses at the same time or give more than your healthcare provider has prescribed.
- If your child does not or cannot take the full dose, call your healthcare provider.
- If you give too much medicine, get emergency medical help right away.
- If your child is able and prefers to swallow the tablets, then you may skip the following steps.
Your pack contains:
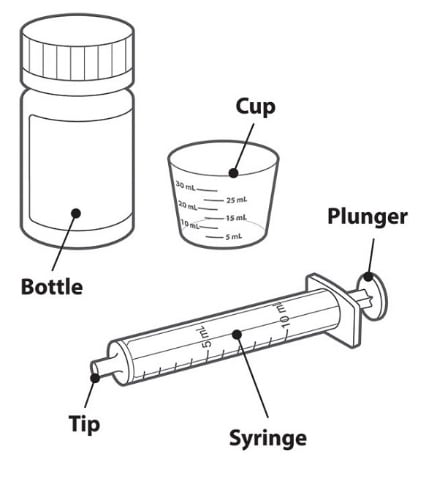
- A bottle containing 60 Tivicay PD tablets for oral suspension.
- Dosing kit:
- Cup: Use this to prepare and give the medicine to children.
- Syringe: Use this to give the medicine to infants.
You will also need:
- Clean drinking water.
Getting Ready
Step 1. Pour water
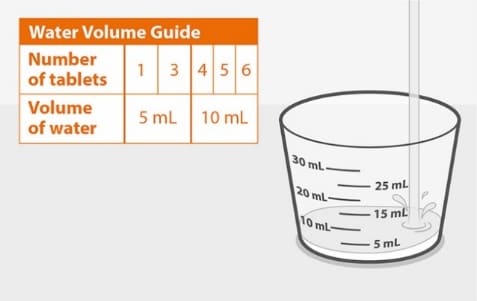
Figure A
- Pour clean drinking water into the cup. The Water Volume Guide in Figure A shows the amount of water needed for the prescribed dose. See Figure A.
Use drinking water only.
Do not use any other drink or food to prepare the dose.
Step 2. Prepare the medicine
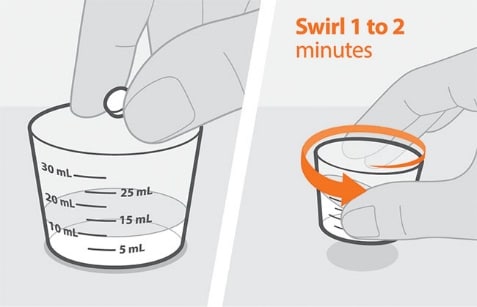
Figure B Figure C
- Add the prescribed number of tablet(s) to the water. See Figure B.
- Swirl the cup gently for 1 to 2 minutes to disperse the tablet(s). The medicine will become cloudy. Take care not to spill any of the medicine. See Figure C.
- Check that the medicine is ready. If there are any lumps of tablet, swirl the cup until they are gone.
If you spill any medicine, clean up the spill.
Throw away the rest of the prepared medicine and make a new dose.
You must give the dose of medicine within 30 minutes of preparing the dose. If it has been more than 30 minutes, wash away all the dose in the cup using water and prepare a new dose of medicine.
Giving the medicine
Step 3. Give the medicine
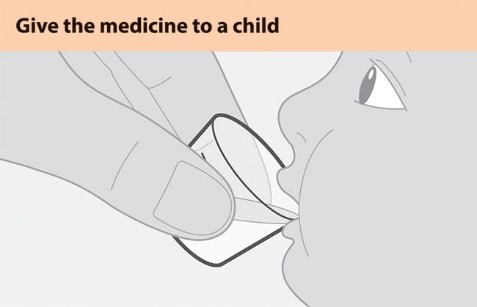
Figure D
- Make sure that the child is upright. Give all the prepared medicine to the child. See Figure D.
- Add another 5 mL of drinking water to the cup, swirl, and give it all to the child.
- Repeat if any medicine remains in the cup to make sure the child gets the full dose.
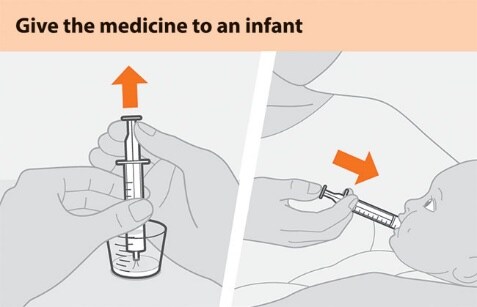
Figure E Figure F
- Place the tip of the syringe into the prepared medicine and draw up all the medicine into the syringe by pulling up on the plunger. See Figure E.
- Place the tip of the syringe against the inside of the infant’s cheek. Gently push down the plunger to give the dose slowly. See Figure F.
- Add another 5 mL of drinking water to the cup and swirl. Draw up the remaining medicine into the syringe and give it all to the infant.
- Repeat if any medicine remains in the syringe to make sure the infant gets the full dose.
Allow time for the medicine to be swallowed.
Cleaning
Step 4. Clean the dosing items
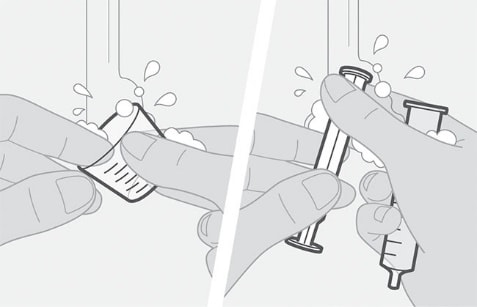
Figure G Figure H
- Wash the cup with water. See Figure G.
- Pull the plunger out of the syringe and wash the syringe parts separately in water. Allow parts to dry completely before reassembling and storing. See Figure H.
- All parts will need to be clean before preparing the next dose.
Storage Information
Store Tivicay PD tablets for oral suspension at room temperature below 86°F (30°C) in the original bottle. Keep the bottle tightly closed and protect from moisture. The bottle contains a desiccant packet to help keep your medicine dry (protect it from moisture). Do not remove the desiccant packet from the bottle.
Keep Tivicay PD and all medicines out of the reach of children.
Label
10 mg
- Each film-coated tablet contains dolutegravir sodium equivalent to 10 mg of dolutegravir.
- 30 Tablets
- Store in original package at controlled room temperature of 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP).
- Keep bottle tightly closed; protect from moisture. Do not remove desiccant.

25 mg
- Each film-coated tablet contains dolutegravir sodium equivalent to 25 mg of dolutegravir.
- 30 Tablets
- Store at controlled room temperature of 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP).

50 mg
- Each film-coated tablet contains dolutegravir sodium equivalent to 50 mg of dolutegravir.
- 30 Tablets
- Store at controlled room temperature of 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP).


Disposal Information
When all the tablets in the bottle have been taken or are no longer needed, throw away the bottle, cup, and syringe. Dispose of them using your local household waste guidelines.
You will get a new cup and syringe in your next pack.
SRC: NLM .