Tepmetko
Generic name: tepotinib
Dosage form: tablets, for oral use
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD
What is Tepmetko?
Tepmetko is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC) that:
- has spread to other parts of the body (metastatic), and
- whose tumors have an abnormal mesenchymal epithelial transition (MET) gene. Your healthcare provider will perform a test to make sure that Tepmetko is right for you.
It is not known if Tepmetko is safe and effective in children.
Description
Tepotinib is a kinase inhibitor. TEPMETKO (tepotinib) tablets for oral use are formulated with tepotinib hydrochloride hydrate. The chemical name for tepotinib hydrochloride hydrate is 3-{1-[(3-{5-[(1-methylpiperidin-4-yl)methoxy]pyrimidin-2-yl}phenyl)methyl]-6-oxo-1,6-dihydropyridazin-3-yl}benzonitrile hydrochloride hydrate. The molecular formula is C29H28N6O2∙HCl∙H2O and the molecular weight is 547.05 g/mol for tepotinib hydrochloride hydrate and 492.58 g/mol for tepotinib (free base). The chemical structure is shown below:
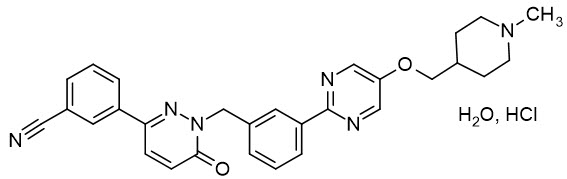
Tepotinib hydrochloride hydrate is a white to off-white powder with a pKa of 9.5.
TEPMETKO is supplied as film-coated tablets containing 225 mg of tepotinib (equivalent to 250 mg tepotinib hydrochloride hydrate). Inactive ingredients in the tablet core are mannitol, microcrystalline cellulose, crospovidone, magnesium stearate, and colloidal silicon dioxide. The tablet coating consists of hypromellose, titanium dioxide, lactose monohydrate, polyethylene glycol, triacetin, and red iron oxides.
Mechanism of Action
Tepotinib is a kinase inhibitor that targets MET, including variants with exon 14 skipping alterations. Tepotinib inhibits hepatocyte growth factor (HGF)-dependent and -independent MET phosphorylation and MET-dependent downstream signaling pathways. Tepotinib also inhibited melatonin 2 and imidazoline 1 receptors at clinically achievable concentrations.
In vitro, tepotinib inhibited tumor cell proliferation, anchorage-independent growth, and migration of MET-dependent tumor cells. In mice implanted with tumor cell lines with oncogenic activation of MET, including METex14 skipping alterations, tepotinib inhibited tumor growth, led to sustained inhibition of MET phosphorylation, and, in one model, decreased the formation of metastases.
What is the most important information I should know about Tepmetko?
Tepmetko may cause serious side effects, including:
- Lung problems. Tepmetko may cause severe or life-threatening swelling (inflammation) of the lungs during treatment that can lead to death. Tell your healthcare provider right away if you develop any new or worsening symptoms of lung problems, including:
- trouble breathing
- shortness of breath
- cough
- fever
See “What are possible side effects of Tepmetko?” for more information about side effects.
What should I tell my healthcare provider before taking Tepmetko?
Before you receive Tepmetko, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had lung or breathing problems other than your lung cancer
- have or have had liver problems
- are pregnant or plan to become pregnant. Tepmetko can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider may do a pregnancy test before you start treatment with Tepmetko.
- You should use effective birth control (contraception) during treatment and for 1 week after the final dose of Tepmetko. Talk to your healthcare provider about birth control methods that may be right for you.
Males with female partners who are able to become pregnant should use effective birth control during treatment with Tepmetko and for 1 week after the final dose of Tepmetko.
- are breastfeeding or plan to breastfeed. It is not known if Tepmetko passes into your breast milk. Do not breastfeed during treatment and for 1 week after the final dose of Tepmetko.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Tepmetko?
- Take Tepmetko exactly as your healthcare provider tells you to.
- Take Tepmetko 1 time a day with food.
- Swallow Tepmetko tablets. Do not chew, crush or split tablets.
- Take your dose of Tepmetko at about the same time each day.
- Do not change your dose or stop taking Tepmetko unless your healthcare provider tells you to.
- If you miss a dose of Tepmetko, take it as soon as you remember. If your next dose is due within 8 hours, skip the missed dose and take your next dose at your regular scheduled time.
- If you vomit after taking a dose of Tepmetko, take your next dose at your regular scheduled time.
Label
PRINCIPAL DISPLAY PANEL – 30 TABLET BLISTER PACK CARTON
- NDC 44087-5000-3
TEPMETKO®
(tepotinib) tablets
225 mg per tablet
Rx Only - Each tablet contains 225 mg of tepotinib
(equivalent to 250 mg tepotinib hydrochloride hydrate) - Each carton contains 3 child resistant blister
cards of 10 tablets each - 30 tablets
EMD
SERONO

What are the possible side effects of Tepmetko?
Tepmetko may cause serious side effects, including:
- See “What is the most important information I should know about Tepmetko?”
- Liver problems. Tepmetko may cause abnormal liver blood test results. Your healthcare provider will do blood tests to check your liver function before you start treatment and during treatment with Tepmetko. Tell your healthcare provider right away if you develop any signs and symptoms of liver problems, including:
- your skin or the white part of your eyes turns yellow
- dark or “tea colored” urine
- light-colored stools (bowel movements)
- confusion
- tiredness
- loss of appetite for several days or longer
- nausea and vomiting
- pain, aching, or tenderness on the right side of your stomach-area (abdomen)
- weakness
- swelling in your stomach-area
The most common side effects of Tepmetko include:
- swelling in your face or other parts of your body
- tiredness
- nausea
- diarrhea
- muscle and joint pain
- shortness of breath
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Tepmetko if you develop serious side effects during treatment.
These are not all of the possible side effects of Tepmetko. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tepmetko
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Tepmetko for a condition for which it was not prescribed. Do not give Tepmetko to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tepmetko that is written for health professionals.
How should I store Tepmetko?
- Store Tepmetko at room temperature between 68°F to 77°F (20°C to 25°C).
- Tepmetko tablets come in blister cards with child-resistant blister foil.
- Store Tepmetko in original package.
Keep Tepmetko and all medicines out of the reach of children.
What are the ingredients in Tepmetko?
Active ingredient: tepotinib
Inactive ingredients: mannitol, microcrystalline cellulose, crospovidone, magnesium stearate, and colloidal silicon dioxide.
Tablet coating: hypromellose, titanium dioxide, lactose monohydrate, polyethylene glycol, triacetin, and red iron oxides.
SRC: NLM .
