Tembexa
Generic name: brincidofovir
Dosage forms: tablets; oral suspension
Medically reviewed by A Ras MD.
What is Tembexa?
Tembexa is a prescription medicine used to treat smallpox disease caused by a type of virus called variola virus in adults, children, and infants.
- The effectiveness of Tembexa has been studied only in animals with orthopoxvirus diseases. There have been no human studies in people who have smallpox disease.
- The safety of Tembexa has been studied in adults and children older than 3 months.
- Tembexa may not work in people who have a weakened immune system.
- The safety and effectiveness of Tembexa is not known for diseases other than human smallpox disease.
Description
TEMBEXA (brincidofovir) tablets, 100 mg, for oral use are immediate release film-coated tablets containing the following inactive ingredients: Colloidal Silicon Dioxide, Crospovidone, FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, Magnesium Stearate, Mannitol, Microcrystalline Cellulose, Polyethylene Glycol, Polyvinyl Alcohol, Purified Water, Silicified Microcrystalline Cellulose, Talc and Titanium Dioxide.
TEMBEXA (brincidofovir) oral suspension, 10 mg/mL, is an aqueous based, preserved, orally dosed suspension. The inactive ingredients are: Citric Acid Anhydrous, Lemon Lime Flavor, Microcrystalline Cellulose and Carboxymethyl Cellulose Sodium, Purified Water, Simethicone 30% Emulsion, Sodium Benzoate, Sucralose, Trisodium Citrate Anhydrous, and Xanthan Gum.
Brincidofovir is an orthopoxvirus nucleotide analog DNA polymerase inhibitor and a lipid conjugate of the nucleotide analog cidofovir and is indicated for the treatment of human smallpox disease. The full chemical name is: Phosphonic acid, P-[[(1S)-2-(4-amino-2-oxo-1(2H)-pyrimidinyl)-1-(hydroxymethyl)ethoxy]methyl]-, mono[3-(hexadecyloxy)propyl] ester.
The molecular formula of brincidofovir is C27H52N3O7P and the relative molecular mass is 561.70.
The structure is shown below.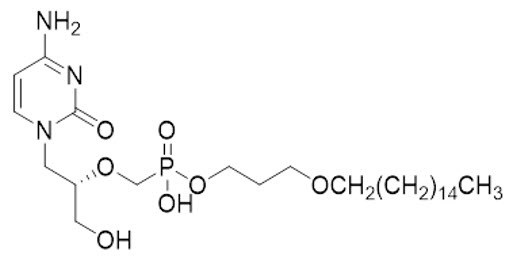
Brincidofovir is a white to off-white crystalline powder as a free acid and practically insoluble in water.
What should I tell my healthcare provider before taking Tembexa?
Before taking Tembexa, tell your healthcare provider about all your medical conditions, including if you:
- are pregnant or plan to become pregnant. Tembexa can harm your unborn baby. Tell your healthcare provider if you become pregnant or think that you may be pregnant during treatment with Tembexa.
- Your healthcare provider should check to see if you are pregnant before you begin treatment with Tembexa.
- Your healthcare provider may use another medicine to treat your smallpox if you are pregnant.
- Individuals who can get pregnant should use effective birth control during treatment with Tembexa and for at least 2 months after the last dose.
- Tembexa may harm your sperm. If you are sexually active with an individual who can become pregnant, you should use condoms during treatment with Tembexa and for at least 4 months after the last dose.
- are breastfeeding or plan to breastfeed. Breastfeeding is not recommended for individuals with smallpox because of the risk of passing variola virus to the breastfed infant.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with Tembexa causing side effects. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Tembexa.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Tembexa with other medicines.
How should I take Tembexa?
- Take Tembexa exactly as your healthcare provider tells you.
- It is important to take Tembexa one time a week for 2 doses (on Day 1 and Day 8), as instructed. Do not miss or skip a dose of Tembexa.
- Tembexa tablets can be taken on an empty stomach or with a low-fat meal (around 400 calories, with about 25% of calories from fat). Talk to your healthcare provider about examples of foods that you can eat for a low-fat meal.
- Tembexa oral suspension can be taken on an empty stomach.
- For adults and children taking the Tembexa oral suspension, shake the suspension bottle well before each use. Use an oral dosing syringe to correctly measure your dose. Ask your pharmacist for an oral dosing syringe if you do not have one. Only take the amount prescribed to you. Throw away (discard) any unused portion.
- For people who are not able to swallow: You may give Tembexa oral suspension through a naso-gastric (NG) or gastrostomy (“g”) tube to someone who is not able to swallow, using the following instructions:
- Draw up the prescribed amount (dose) of Tembexa oral suspension using a catheter-tip syringe with mL markings on it.
- Give the dose through the naso-gastric tube or gastrostomy tube.
- Refill the catheter-tip syringe with 3 mL of water and shake the syringe. Give the contents of the syringe through the naso-gastric tube or gastrostomy tube.
- Flush with water before and after administration.
- Stay under the care of your healthcare provider during treatment with Tembexa.
- Do not change your dose or stop taking Tembexa without talking to your healthcare provider.
- Swallow Tembexa tablets whole. Do not divide, break, or crush Tembexa tablets. Do not touch broken or crushed tablets or oral suspension. If you touch Tembexa, wash your hands very well with soap and water. If you get Tembexa in your eyes, rinse your eyes well with water.
- If you take too much Tembexa, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Tembexa?
Tembexa may cause serious side effects, including:
- Liver problems. Your healthcare provider should perform blood tests to check your liver before you start taking Tembexa and during treatment with Tembexa for any signs or symptoms of liver problems. Call your healthcare provider right away if you have the following symptoms:
- Stomach discomfort in the upper right side
- Dark urine
- Yellowing of your skin or the whites of your eyes (jaundice)
- Diarrhea. Diarrhea is common in people who take Tembexa but can also be serious. Call your healthcare provider right away if you develop diarrhea with 4 or more stools per day over your usual daily number of stools.
The most common side effects of Tembexa include:
Tembexa may cause low sperm counts and affect the ability to conceive children. Talk to your healthcare provider if you have concerns about fertility. These are not all the possible side effects of Tembexa. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tembexa
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Tembexa for a condition for which it was not prescribed. Do not give Tembexa to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tembexa that is written for health professionals.
How should I store Tembexa?
- Store Tembexa tablets and oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not freeze Tembexa oral suspension.
- Keep Tembexa in its original container.
Keep Tembexa and all other medicines out of the reach of children.
What are the ingredients in Tembexa?
Active ingredient: brincidofovir
Inactive ingredients:
Tablets: Colloidal Silicon Dioxide, Crospovidone, FD&C Blue #1/Brilliant Blue FCF Aluminum Lake, FD&C Blue #2/Indigo Carmine Aluminum Lake, Magnesium Stearate, Mannitol, Microcrystalline Cellulose, Polyethylene Glycol, Polyvinyl Alcohol, Purified Water, Silicified Microcrystalline Cellulose, Talc, Titanium Dioxide.
Oral Suspension: Citric Acid Anhydrous, Lemon Lime Flavor, Microcrystalline Cellulose and Carboxymethyl Cellulose Sodium, Purified Water, Simethicone 30% Emulsion, Sodium Benzoate, Sucralose, Trisodium Citrate Anhydrous, Xanthan Gum.
Label
PRINCIPAL DISPLAY PANEL – ORAL SUSPENSION CARTON LABEL
- For oral use
- Rx Only
- TEMBEXA®
brincidofovir - Oral Suspension, 10 mg/mL
- Contents: 65 mL
- See Full
Prescribing Information
For TEMBEXA Inside. - NDC 79622-012-65

SRC: NLM .
