Tekturna
Generic name: aliskiren
Drug class: Renin inhibitors
Medically reviewed by A Ras MD
What is Tekturna?
Tekturna is a prescription medicine used to treat high blood pressure (hypertension) in adults and children weighing 50 kg or greater who are at least 6 years of age to lower blood pressure.
It is not known if Tekturna is safe and effective in children under 6 years of age.
Description
Tekturna contains aliskiren hemifumarate, adirect renin inhibitor. Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy- 2,7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate and its structural formula is:
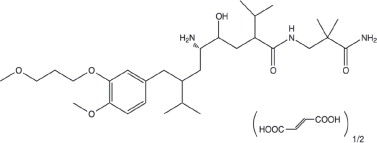
Molecular formula: C30H53N3O6 • 0.5 C4H4O4
Aliskiren hemifumarate is a white to slightly yellowish crystalline powder with a molecular weight of 609.8 (free base- 551.8). It is soluble in phosphate buffer, n-octanol, and highly soluble in water.
Tekturna is available as film-coated tablets, which contains 165.75 mg or 331.5 mg aliskiren hemifumerate (equivalent to 150 mg or 300 mg aliskiren) and the following excipients: crospovidone; magnesium stearate; microcrystalline cellulose; povidone; silica, colloidal anhydrous; hypromellose; macrogol; talc; iron oxide, black (E 172); iron oxide, red (E 172); titanium dioxide (E 171).
Mechanism of Action
Renin is secreted by the kidney in response to decreases in blood volume and renal perfusion. Renin cleaves angiotensinogen to form the inactive decapeptide angiotensin I (Ang I). Ang I is converted to the active octapeptide angiotensin II (Ang II) by ACE and non-ACE pathways. Ang II is a powerful vasoconstrictor and leads to the release of catecholamines from the adrenal medulla and prejunctional nerve endings. It also promotes aldosterone secretion and sodium reabsorption.
Together, these effects increase blood pressure. Ang II also inhibits renin release, thus providing a negative feedback to the system. This cycle, from renin through angiotensin to aldosterone and its associated negative feedback loop, is known as the renin-angiotensin-aldosterone system (RAAS). Aliskiren is a direct renin inhibitor, decreasing plasma renin activity (PRA) and inhibiting the conversion of angiotensinogen to Ang I. Whether aliskiren affects other RAAS components, e.g., ACE or non-ACE pathways, is not known.
All agents that inhibit the RAAS, including renin inhibitors, suppress the negative feedback loop, leading to a compensatory rise in plasma renin concentration. When this rise occurs during treatment with ACEIs and ARBs, the result is increased levels of PRA. During treatment with aliskiren, however, the effect of increased renin levels is blocked so that PRA, Ang I and Ang II are all reduced, whether aliskiren is used as monotherapy or in combination with other antihypertensive agents.
What is the most important information I should know about Tekturna?
Tekturna can cause harm or death to your unborn baby.
- Talk to your doctor about other ways to lower your blood pressure if you plan to become pregnant.
- If you become pregnant during treatment with Tekturna, stop taking Tekturna and tell your doctor right away.
What is high blood pressure (hypertension)?
Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too great. High blood pressure makes the heart work harder to pump blood through the body and causes damage to the blood vessels. Tekturna can help your blood vessels relax so your blood pressure is lower. Medicines that lower your blood pressure lower your chance of having a stroke or heart attack.
Who should not take Tekturna?
Do not take Tekturna if:
- you have diabetes and are taking a kind of medicine called an angiotensin receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI).
- you are allergic to any of the ingredients in Tekturna. See the end of this leaflet for a complete list of the ingredients in Tekturna.
Do not give Tekturna to children less than 2 years of age.
What should I tell my healthcare provider before taking Tekturna?
Before taking Tekturna, tell your doctor about all of your medical conditions, including if you:
- have kidney or heart problems.
- have diabetes.
- have ever had an allergic reaction to another blood pressure medicine.
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Tekturna?”
- are breastfeeding or plan to breastfeed. It is not known if Tekturna passes into your breast milk. You should not breastfeed during treatment with Tekturna.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Especially tell your doctor if you are taking:
- a kind of medicine to control blood pressure called angiotensin receptor blocker (ARB) or angiotensin-converting enzyme inhibitor (ACEI).
- water pills (also called “diuretics”).
- cyclosporine (Gengraf, Neoral, Sandimmune)
- itraconazole (Onmel, Sporanox).
- potassium-containing medicines, potassium supplements, or salt substitutes containing potassium.
- nonsteroidal anti-inflammatory drugs (NSAIDs).
Ask your doctor if you are not sure if you are taking one of the medicines listed above.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist when you get a new medicine. Your doctor or pharmacist will know what medicines are safe to take together.
How should I take Tekturna?
- Take Tekturna exactly as your doctor tells you to take it.
- Take Tekturna 1 time a day, about the same time each day.
- Your doctor may change your dose if needed.
What are the possible side effects of Tekturna?
Tekturna may cause serious side effects, including:
- See “What is the most important information I should know about Tekturna?”
- Serious allergic reactions that can be life threatening. Stop taking Tekturna and get emergency medical help right away and if you get any of these symptoms of a serious reactions during treatment with Tekturna:
- trouble breathing or swallowing
- chest tightness
- raised bumps (hives)
- rash
- swelling of the face, lips, tongue, throat, arms, legs or the whole body
- itching
- dizziness
- vomiting
- stomach (abdomen) pain
- Low blood pressure (hypotension). Your blood pressure may get too low if you also take water pills, are on a low-salt diet, sweat more than normal, receive dialysis treatments, have heart problems, or have vomiting or diarrhea. Lie down if you feel faint or dizzy and call your doctor right away.
- Kidney problems, including kidney failure. Tell your doctor if you urinate less.
- Increased potassium blood levels (hyperkalemia). Your doctor will check your potassium blood level during treatment with Tekturna.
The most common side effect of Tekturna is diarrhea.
These are not all of the possible side effects of Tekturna.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tekturna
Medicines are sometimes prescribed for purpose other than those listed in the Patient Information leaflet. Do not take Tekturna for a condition for which it was not prescribed. Do not give Tekturna to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information that is written for healthcare professionals.
How should I store Tekturna?
- Store Tekturna at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Tekturna in the original container it comes in. Do not remove the desiccant (drying agent) from the bottle.
- Protect Tekturna from moisture.
Keep Tekturna and all medicines out of the reach of children.
Label
Principal Display Panel – Package Label – 150 mg Bottle Label
- NDC 70839-150-30 30 tablets
- Tekturna®
- (aliskiren)
- Tablets
- Rx only 150 mg
- NODEN PHARMA™

Principal Display Panel – Package Label – 300 mg Bottle Label
- NDC 70839-300-30 30 tablets
- Tekturna®
- (aliskiren)
- Tablets
- Rx only 300 mg
- NODEN PHARMA™

What are the ingredients in Tekturna?
Tekturna is available as film-coated tablets, which contains 165.75 mg or 331.5 mg aliskiren hemifumerate (equivalent to 150 mg or 300 mg aliskiren).
Active ingredient: aliskiren hemifumarate
Tekturna tablets inactive ingredients: crospovidone, magnesium stearate, microcrystalline cellulose, povidone, silica, colloidal anhydrous, hypromellose, macrogol, talc, iron oxide, black (E172), iron oxide, red (E172), and titanium dioxide (E171).
SRC: NLM .
