Tazverik
Generic name: tazemetostat
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Tazverik?
Tazverik is a prescription medicine used to treat adults and children aged 16 years and older with epithelioid sarcoma that has spread or grown and cannot be removed by surgery. this is also used in adults with follicular lymphoma when the disease has come back or did not respond to treatment, whose tumors have an abnormal EZH2 gene, and who have been treated with at least two prior medicines. Tazverik is also used in adults with follicular lymphoma when the disease has come back or did not respond to treatment, who have no other satisfactory treatment options.
It is not known if Tazverik is safe and effective in children less than 16 years of age.
Description
Tazemetostat is a methyltransferase inhibitor. Tazemetostat hydrobromide has the following chemical name: [1,1′-Biphenyl]-3-carboxamide, N-[(1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl]-5-[ethyl(tetrahydro-2H-pyran-4-yl)amino]-4-methyl-4′-(4-morpholinylmethyl)-, hydrobromide (1:1). The molecular formula of tazemetostat hydrobromide is C34H44N4O4∙HBr. Tazemetostat hydrobromide has a molecular weight of 653.66 g/mol and the following structural formula:
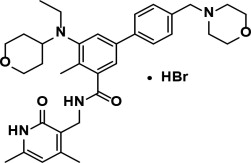
Tazemetostat hydrobromide is a white to off-white solid that is slightly soluble in water and has pKa values of 5.26, 6.88, and 12.62. A saturated aqueous solution of tazemetostat hydrobromide has a pH of approximately 5 at ambient conditions.
TAZVERIK (tazemetostat) tablets for oral use contain 200 mg tazemetostat, equivalent to 228 mg tazemetostat hydrobromide.
Each tablet is film-coated and contains the following inactive ingredients in the tablet core: hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, and sodium starch glycolate. The film-coat contains hypromellose, polyethylene glycol, red iron oxide, talc, and titanium dioxide.
Mechanism of Action
Tazemetostat is an inhibitor of the methyltransferase, EZH2, and some EZH2 gain-of-function mutations including Y646X, A682G, and A692V. Tazemetostat also inhibited EZH1 with a half-maximal inhibitory concentration (IC50) of 392 nM, approximately 36 times higher than the IC50 for inhibition of EZH2.
The most well-characterized function of EZH2 is as the catalytic subunit of the polycomb repressive complex 2 (PRC2), catalyzing mono-, di-, and trimethylation of lysine 27 of histone H3. Trimethylation of histone H3 leads to transcriptional repression.
SWItch/Sucrose Non-Fermentable (SWI/SNF) complexes can antagonize PRC2 function in the regulation of the expression of certain genes of patients with epithelioid sarcoma. Preclinical in vitro and in vivo models with the loss or dysfunction of certain SWI/SNF complex members (e.g., integrase interactor 1 [INI1/SNF5/SMARCB1/BAF47], SMARCA4, and SMARCA2) can lead to aberrant EZH2 activity or expression and a resulting oncogenic dependence on EZH2.
Tazemetostat suppressed proliferation of B-cell lymphoma cell lines in vitro and demonstrated antitumor activity in a mouse xenograft model of B-cell lymphoma with or without EZH2 gain-of-function mutations. Tazemetostat demonstrated greater effects on the inhibition of proliferation of lymphoma cell lines with mutant EZH2.
What is the most important information I should know about Tazverik?
Tazverik can cause serious side effects, including:
- Risk of new cancers. An increase in new (second) cancers has happened in people who were treated with Tazverik. Talk with your healthcare provider about your risk of developing new cancers. Your healthcare provider will monitor you for new cancers after your treatment with Tazverik. Tell your healthcare provider if you are more tired than usual, or have easy bruising, fever, bone pain, or paleness.
See “What are the possible side effects of Tazverik” for more information about side effects.
What should I tell my healthcare provider before taking Tazverik?
Before taking Tazverik tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. Tazverik can harm your unborn baby. Your healthcare provider will give you a pregnancy test before you start treatment with Tazverik. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- Females who are able to become pregnant should use effective non-hormonal birth control (such as condoms) during treatment and for 6 months after the final dose of Tazverik. Birth control pills (oral contraceptives) and other hormonal forms of birth control may not be effective if used during treatment with Tazverik. Talk to your healthcare provider about birth control options that are right for you.
- Males with female partners who are able to become pregnant should use effective birth control during treatment and for 3 months after the final dose of Tazverik.
- are breastfeeding or plan to breastfeed. It is not known if Tazverik passes into your breast milk. Do not breastfeed during treatment and for 1 week after the final dose of Tazverik.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tazverik may affect the way other medicines work and other medicines may affect how Tazverik works.
How should I take Tazverik?
- Take Tazverik exactly as your healthcare provider tells you.
- Take Tazverik 2 times each day.
- Take Tazverik with or without food.
- Swallow Tazverik tablets whole. Do not cut, crush, or chew tablets.
- If you miss a dose or vomit after taking your dose, just skip that dose and take the next dose at your regular time.
- Your healthcare provider may change your dose, temporarily stop, or completely stop treatment with Tazverik if you get certain side effects.
What should I avoid while taking Tazverik?
- Avoid eating grapefruit or drinking grapefruit juice during treatment with Tazverik.
- Avoid taking St. John’s wort during treatment with Tazverik.
What are the possible side effects of Tazverik?
Tazverik can cause serious side effects. See “What is the most important information I should know about Tazverik?”
The most common side effects of Tazverik in people with epithelioid sarcoma include:
- pain
- nausea
- vomiting
- tiredness
- decreased appetite
- constipation
The most common side effects of Tazverik in people with follicular lymphoma include:
- tiredness
- bone and muscle pain
- cold-like symptoms (upper respiratory infection)
- nausea
- stomach (abdominal) pain
These are not all the possible side effects of Tazverik.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tazverik
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Tazverik for a condition for which it was not prescribed. Do not give Tazverik to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tazverik that is written for health professionals.
How should I store Tazverik?
Do not store Tazverik tablets above 86°F (30°C).
Keep Tazverik and all medicines out of the reach of children.
What are the ingredients in Tazverik?
Active Ingredient: tazemetostat.
Inactive Ingredients: Tablet core: hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, and sodium starch glycolate. Film-coating: hypromellose, polyethylene glycol, red iron oxide, talc, and titanium dioxide.
What is Tazverik?
Tazverik is a prescription medicine used to treat:
- adults and children aged 16 years and older with epithelioid sarcoma that has spread or grown and cannot be removed by surgery.
- adults with follicular lymphoma when the disease has come back or did not respond to treatment, whose tumors have an abnormal EZH2 gene, and who have been treated with at least two prior medicines. Your healthcare provider will perform a test to make sure Tazverik is right for you.
- adults with follicular lymphoma when the disease has come back or did not respond to treatment, who have no other satisfactory treatment options.
It is not known if Tazverik is safe and effective in children less than 16 years of age.
What is the most important information I should know about Tazverik?
Tazverik can cause serious side effects, including:
- Risk of new cancers. An increase in new (second) cancers has happened in people who were treated with Tazverik. Talk with your healthcare provider about your risk of developing new cancers. Your healthcare provider will monitor you for new cancers after your treatment with Tazverik. Tell your healthcare provider if you are more tired than usual, or have easy bruising, fever, bone pain, or paleness.
See “What are the possible side effects of Tazverik” for more information about side effects.
What should I tell my healthcare provider before taking Tazverik?
Before taking Tazverik tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. Tazverik can harm your unborn baby. Your healthcare provider will give you a pregnancy test before you start treatment with Tazverik. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
- Females who are able to become pregnant should use effective non-hormonal birth control (such as condoms) during treatment and for 6 months after the final dose of Tazverik. Birth control pills (oral contraceptives) and other hormonal forms of birth control may not be effective if used during treatment with Tazverik. Talk to your healthcare provider about birth control options that are right for you.
- Males with female partners who are able to become pregnant should use effective birth control during treatment and for 3 months after the final dose of Tazverik.
- are breastfeeding or plan to breastfeed. It is not known if Tazverik passes into your breast milk. Do not breastfeed during treatment and for 1 week after the final dose of Tazverik.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Tazverik may affect the way other medicines work and other medicines may affect how Tazverik works.
How should I take Tazverik?
- Take Tazverik exactly as your healthcare provider tells you.
- Take Tazverik 2 times each day.
- Take Tazverik with or without food.
- Swallow Tazverik tablets whole. Do not cut, crush, or chew tablets.
- If you miss a dose or vomit after taking your dose, just skip that dose and take the next dose at your regular time.
- Your healthcare provider may change your dose, temporarily stop, or completely stop treatment with Tazverik if you get certain side effects.
What should I avoid while taking Tazverik?
- Avoid eating grapefruit or drinking grapefruit juice during treatment with Tazverik.
- Avoid taking St. John’s wort during treatment with Tazverik.
What are the possible side effects of Tazverik?
Tazverik can cause serious side effects. See “What is the most important information I should know about Tazverik?”
The most common side effects of Tazverik in people with epithelioid sarcoma include:
- pain
- nausea
- vomiting
- tiredness
- decreased appetite
- constipation
The most common side effects of Tazverik in people with follicular lymphoma include:
- tiredness
- bone and muscle pain
- cold-like symptoms (upper respiratory infection)
- nausea
- stomach (abdominal) pain
These are not all the possible side effects of Tazverik.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Tazverik Images
-
Tazverik 200 mg
General information about the safe and effective use of Tazverik
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Tazverik for a condition for which it was not prescribed. Do not give Tazverik to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tazverik that is written for health professionals.
How should I store Tazverik?
Do not store Tazverik tablets above 86°F (30°C).
Keep Tazverik and all medicines out of the reach of children.
What are the ingredients in Tazverik?
Active Ingredient: tazemetostat.
Inactive Ingredients: Tablet core: hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, and sodium starch glycolate. Film-coating: hypromellose, polyethylene glycol, red iron oxide, talc, and titanium dioxide.
Label
Principal Display Panel – 200 mg Bottle Label
- NDC 72607-100-00 240 Tablets
- TAZVERIK™
- (tazemetostat) tablets
- 200 mg
- Rx only
- Dispense the Medication Guide, attached or provided separately, to each patient pursuant to Federal Law.
- Epizyme

 SRC: NLM .
SRC: NLM .