Taxotere
Generic name: docetaxel
Drug class: Mitotic inhibitors
Medically reviewed by A Ras MD.
What is Taxotere?
Taxotere is a prescription anti-cancer medicine used to treat certain people with, breast cancer, non-small cell lung cancer, prostate cancer, stomach cancer, head and neck cancer
It is not known if Taxotere is effective in children.
Description
Docetaxel is an antineoplastic agent belonging to the taxoid family. It is prepared by semisynthesis beginning with a precursor extracted from the renewable needle biomass of yew plants. The chemical name for docetaxel is (2R,3S)-N-carboxy-3-phenylisoserine,N- tert-butyl ester, 13-ester with 5β-20-epoxy-1,2α,4,7β,10β,13α-hexahydroxytax-11-en-9-one 4-acetate 2-benzoate. Docetaxel (anhydrous) has the following structural formula:
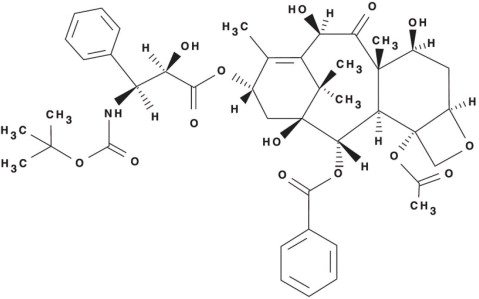
Docetaxel is a white to almost-white powder with an empirical formula of C 43H 53NO 14, and a molecular weight of 807.88. It is highly lipophilic and practically insoluble in water.
Docetaxel Injection, USP is a sterile, nonpyrogenic, clear, colorless to pale yellow solution at 10 mg per mL concentration.
Each mL contains 10 mg Docetaxel (anhydrous), USP, 260 mg Polysorbate 80 NF, 4 mg Anhydrous Citric Acid USP, 23% v/v Dehydrated Alcohol USP, and Polyethylene Glycol 300 NF.
Docetaxel Injection, USP is available in multi-dose vials containing 80 mg (8 mL) or 160 mg (16 mL) docetaxel (anhydrous).
Docetaxel Injection, USP requires NO prior dilution with a diluent and is ready to add to the infusion solution.
What is the most important information I should know about Taxotere?
Taxotere can cause serious side effects, including death.
- The chance of death in people who receive Taxotere is higher if you:
- have liver problems
- receive high doses of Taxotere
- have non-small cell lung cancer and have been treated with chemotherapy medicines that contain platinum
- Taxotere can affect your blood cells. Your healthcare provider should do routine blood tests during treatment with Taxotere. This will include regular checks of your white blood cell counts. If your white blood cells are too low, your healthcare provider may not treat you with Taxotere until you have enough white blood cells. People with low white blood cell counts can develop life-threatening infections. The earliest sign of infection may be fever. Follow your healthcare provider’s instructions for how often to take your temperature during treatment with Taxotere. Call your healthcare provider right away if you have a fever.
- Swelling (inflammation) of the small intestine and colon. This can happen at any time during treatment and could lead to death as early as the first day you get symptoms. Tell your healthcare provider right away if you develop new or worse symptoms of intestinal problems, including stomach (abdominal) pain or tenderness or diarrhea, with or without fever.
- Severe allergic reactions are medical emergencies that can happen in people who receive Taxotere and can lead to death. You may be at higher risk of developing a severe allergic reaction to Taxotere if you are allergic to paclitaxel. Your healthcare provider will monitor you closely for allergic reactions during your Taxotere infusion.
Tell your healthcare provider right away if you have any of these signs of a severe allergic reaction:- trouble breathing
- sudden swelling of your face, lips, tongue, throat, or trouble swallowing
- hives (raised bumps), rash, or redness all over your body
- Your body may hold too much fluid (severe fluid retention) during treatment with Taxotere. This can be life threatening. To decrease the chance of this happening, you must take another medicine, a corticosteroid, before each Taxotere treatment. You must take the corticosteroid exactly as your healthcare provider tells you. Tell your healthcare provider or nurse before your Taxotere treatment if you forgot to take your corticosteroid dose or do not take it as your healthcare provider tells you. Tell your healthcare provider right away if you have swelling in your legs or feet, weight gain or shortness of breath.
- Risk of new cancers. An increase in new (second) cancers has happened in people treated with Taxotere together with certain other anticancer treatments. This includes certain blood cancers, such as acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), non-Hodgkin’s Lymphoma (NHL), and kidney cancer.
- Changes in blood counts due to leukemia and other blood disorders may occur years after treatment with Taxotere.
Your healthcare provider will check you for new cancers during and after your treatment with Taxotere.
- Changes in blood counts due to leukemia and other blood disorders may occur years after treatment with Taxotere.
- Severe skin problems. Tell your healthcare provider right away if you have any of these signs of a severe skin reaction:
- redness and swelling of your arms and legs.
- blistering, peeling, or bleeding on any part of your skin (including your lips, eyes, mouth, nose, genitals, hands or feet) with or without a rash. You may also have flu-like symptoms such as fever, chills, or muscle aches.
- red, scaly rash all over your body with blisters, small red or white bumps under the skin that contain pus (pustules), and fever.
Who should not take Taxotere?
Do not receive Taxotere if you:
- have a low white blood cell count.
- have had a severe allergic reaction to:
- docetaxel, the active ingredient in Taxotere, or
- any other medicines that contain polysorbate 80. Ask your healthcare provider or pharmacist if you are not sure.
See “What is the most important information I should know about Taxotere?” for the signs and symptoms of a severe allergic reaction.
See the end of this Patient Information for a complete list of the ingredients in Taxotere.
What should I tell my healthcare provider before taking Taxotere?
Before you receive Taxotere, tell your healthcare provider about all of your medical conditions, including if you:
- are allergic to any medicines, including paclitaxel. See “Who should not take Taxotere?”.
- have liver problems
- are pregnant or plan to become pregnant. Taxotere can harm your unborn baby. You should not become pregnant during treatment with Taxotere. Tell your healthcare provider if you become pregnant or you think you may be pregnant during treatment with Taxotere.
Females who are able to become pregnant:- Your healthcare provider will check to see if you are pregnant before you start treatment with Taxotere.
- You should use effective birth control (contraception) during treatment with Taxotere and for 6 months after the last dose.
- Males with female partners who are able to become pregnant should use effective birth control during treatment with Taxotere and for 3 months after the last dose.Talk to your healthcare provider if you have questions about birth control options that are right for you.
- are breastfeeding or plan to breastfeed. It is not known if Taxotere passes into your breast milk. Do not breastfeed during treatment with Taxotere and for 1 week after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taxotere may affect the way other medicines work, and other medicines may affect the way Taxotere works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Taxotere?
- Taxotere will be given to you as an intravenous (IV) injection into your vein, usually over 1 hour.
- Taxotere is usually given every 3 weeks.
- Your healthcare provider will decide how long you will receive treatment with Taxotere.
- Your healthcare provider will check your blood cell counts and other blood tests during your treatment with Taxotere to check for side effects of Taxotere.
- Your healthcare provider may stop your treatment, change the timing of your treatment, or change the dose of your treatment if you have certain side effects while receiving Taxotere.
What are the possible side effects of Taxotere?
Taxotere may cause serious side effects including death.
- See ”What is the most important information I should know about Taxotere?”
- Neurologic problems. Neurologic symptoms are common in people who receive Taxotere but can be severe. Tell your healthcare provider right away if you have numbness, tingling, or burning in your hands or feet (peripheral neuropathy) or weakness of your legs, feet, arms, or hands (motor weakness).
- Vision problems including blurred vision or loss of vision. Tell your healthcare provider right away if you have any vision changes.
- Taxotere injection contains alcohol. The alcohol content in Taxotere may impair your ability to drive or use machinery right after receiving Taxotere. Consider whether you should drive, operate machinery or do other dangerous activities right after you receive Taxotere treatment.
- You may experience side effects of this medicine that may impair your ability to drive, use tools, or operate machines. If this happens, do not drive or use any tools or machines before discussing with your healthcare provider.
The most common side effects of Taxotere include:
- infections
- low white blood cells (help fight infections), low red blood cells (anemia) and low platelets (help blood to clot)
- allergic reactions (See “What is the most important information I should know about Taxotere?”)
- changes in your sense of taste
- shortness of breath
- constipation
- decreased appetite
- changes in your fingernails or toenails
- swelling of your hands, face, or feet
- feeling weak or tired
- joint and muscle pain
- nausea and vomiting
- diarrhea
- mouth or lip sores
- hair loss: in some people, permanent hair loss has been reported
- redness of the eye, excess tearing
- skin reactions at the site of Taxotere administration such as increased skin pigmentation, redness, tenderness, swelling, warmth or dryness of the skin
- tissue damage if Taxotere leaks out of the vein into the tissues
Tell your healthcare provider if you have a fast or irregular heartbeat, severe shortness of breath, dizziness or fainting during your infusion. If any of these events occurs after your infusion, get medical help right away.
Taxotere may affect fertility in males. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of Taxotere. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Taxotere
Medicines are sometimes prescribed for purposes other than those listed in this Patient Information. You can ask your pharmacist or healthcare provider for information about Taxotere that is written for health professionals.
How should I store Taxotere?
Store between 2°C and 25°C (36°F and 77°F). Retain in the original package to protect from light. Freezing does not adversely affect the product.
What are the ingredients in Taxotere?
Active ingredient: docetaxel
Inactive ingredients: polysorbate 80 and dehydrated alcohol solution.
SRC: NLM .
