Tafinlar
Generic name: dabrafenib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Tafinlar used for?
Tafinlar is Prescription medicine that he used to treat melanoma. Also used in the treatment of lung cancer.
Description
Dabrafenib mesylate is a kinase inhibitor. The chemical name for dabrafenib mesylate is N-{3-[5-(2-amino-4-pyrimidinyl)-2-(1,1-dimethylethyl)-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzene sulfonamide, methanesulfonate salt. It has the molecular formula C23H20F3N5O2S2•CH4O3S and a molecular weight of 615.68 g/mol. Dabrafenib mesylate has the following chemical structure:
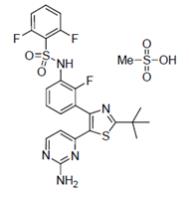
Dabrafenib mesylate is a white to slightly colored solid with three pKas: 6.6, 2.2, and -1.5. It is very slightly soluble at pH 1 and practically insoluble above pH 4 in aqueous media.
TAFINLAR (dabrafenib) capsules for oral use are supplied as 50 mg and 75 mg capsules for oral administration. Each 50 mg capsule contains 59.25 mg dabrafenib mesylate equivalent to 50 mg of dabrafenib free base. Each 75 mg capsule contains 88.88 mg dabrafenib mesylate equivalent to 75 mg of dabrafenib free base. The inactive ingredients of TAFINLAR are colloidal silicon dioxide, magnesium stearate, and microcrystalline cellulose. Capsule shells contain hypromellose, red iron oxide (E172), and titanium dioxide (E171).
Mechanism of Action
Dabrafenib is an inhibitor of some mutated forms of BRAF kinases with in vitro IC50 values of 0.65, 0.5, and 1.84 nM for BRAF V600E, BRAF V600K, and BRAF V600D enzymes, respectively. Dabrafenib also inhibits wild-type BRAF and CRAF kinases with IC50 values of 3.2 and 5.0 nM, respectively, and other kinases, such as SIK1, NEK11, and LIMK1 at higher concentrations. Some mutations in the BRAF gene, including those that result in BRAF V600E, can result in constitutively activated BRAF kinases that may stimulate tumor cell growth [see Indications and Usage (1)]. Dabrafenib inhibits cell growth of various BRAF V600 mutation-positive tumors in vitro and in vivo.
Dabrafenib and trametinib target two different kinases in the RAS/RAF/MEK/ERK pathway. Use of dabrafenib and trametinib in combination resulted in greater growth inhibition of BRAF V600 mutation-positive tumor cell lines in vitro and prolonged inhibition of tumor growth in BRAF V600 mutation positive tumor xenografts compared with either drug alone.
Before taking Tafinlar, tell your doctor:
- If you are allergic to Tafinlar; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you are breast-feeding. Do not breast-feed while you take Tafinlar and for 2 weeks after your last dose.
This medicine may interact with other drugs or health problems.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Tafinlar with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Tafinlar?
- Tell all of your health care providers that you take Tafinlar. This includes your doctors, nurses, pharmacists, and dentists.
- Sometimes, dabrafenib is taken with trametinib. If you are taking dabrafenib with trametinib, be sure you know the side effects that can happen with each drug. When these drugs are taken together, the chance of certain side effects may be raised. These side effects can be very bad and sometimes deadly. This includes bleeding, bleeding in the brain, blood clots, eye problems, fever, heart problems (like heart failure), high blood sugar, other cancers, and skin problems. Talk with your doctor about the chance of side effects with your drugs.
- This medicine may add to the chance of getting some types of cancer. Talk with the doctor.
- Have your skin checked. Tell your doctor if you have any skin changes like a new wart, skin sore or reddish bump that bleeds or does not heal, or a change in the color or size of a mole.
- Have blood work checked as you have been told by the doctor. Talk with the doctor.
- If you have high blood sugar (diabetes), talk with your doctor. This medicine may raise blood sugar.
- Check your blood sugar as you have been told by your doctor.
- It is common to get a rash with Tafinlar but other skin reactions may also happen. Sometimes, these rashes and skin reactions can be very bad and may need treatment in the hospital. Call your doctor right away if you have acne, skin redness, or a skin rash that bothers you or does not go away. Call your doctor right away if you have redness or irritation of the palms of hands or soles of feet.
- Very bad eye problems have happened with Tafinlar. Sometimes, this has led to loss of eyesight. Call your doctor right away if you have blurred eyesight, loss of eyesight, or other changes in eyesight. Call your doctor right away if you see color dots or halos or if bright lights bother you.
- Be careful if you have G6PD deficiency. Anemia may happen.
- If you are 65 or older, use Tafinlar with care. You could have more side effects.
- This medicine may affect fertility. Fertility problems may lead to not being able to get pregnant or father a child.
- This medicine may cause harm to an unborn baby. A pregnancy test will be done before you start Tafinlar to show that you are NOT pregnant.
- Use a non-hormone type of birth control like condoms to prevent pregnancy while taking Tafinlar and for at least 2 weeks after stopping Tafinlar. Birth control pills and other hormone-based birth control may not work as well to prevent pregnancy.
- If you get pregnant while taking Tafinlar or within 2 weeks after your last dose, call your doctor right away.
- Men with a partner who is pregnant or may get pregnant must use a condom while taking Tafinlar and for some time after the last dose. Use a condom even if you have had a vasectomy. Ask your doctor how long to use a condom. If your partner is pregnant or gets pregnant, call the doctor right away.
How is Tafinlar best taken?
Use Tafinlar as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take on an empty stomach. Take 1 hour before or 2 hours after meals.
- Swallow whole. Do not chew, open, or crush.
- Talk with your doctor about drinking lots of fluids and other ways to prevent fluid loss. If you have a lot of fluid loss, you may have more side effects from Tafinlar.
- Keep taking Tafinlar as you have been told by your doctor or other health care provider, even if you feel well.
What do I do if I miss a dose?
- Take a missed dose as soon as you think about it.
- If it is less than 6 hours until the next dose, skip the missed dose and go back to the normal time.
- Do not take 2 doses at the same time or extra doses.
What are the side effects of Tafinlar that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Signs of bleeding like throwing up or coughing up blood; vomit that looks like coffee grounds; blood in the urine; black, red, or tarry stools; bleeding from the gums; abnormal vaginal bleeding; bruises without a cause or that get bigger; or bleeding you cannot stop.
- Signs of fluid and electrolyte problems like mood changes, confusion, muscle pain or weakness, a heartbeat that does not feel normal, very bad dizziness or passing out, fast heartbeat, more thirst, seizures, feeling very tired or weak, not hungry, unable to pass urine or change in the amount of urine produced, dry mouth, dry eyes, or very bad upset stomach or throwing up.
- Signs of high blood sugar like confusion, feeling sleepy, more thirst, more hungry, passing urine more often, flushing, fast breathing, or breath that smells like fruit.
- Signs of kidney problems like unable to pass urine, change in how much urine is passed, blood in the urine, or a big weight gain.
- Weakness on 1 side of the body, trouble speaking or thinking, change in balance, drooping on one side of the face, or blurred eyesight.
- Fever or chills.
- Dark urine or yellow skin or eyes.
- Eye pain.
- Chest pain or pressure.
- This medicine may cause heart failure. You will need to have your heart function checked while taking Tafinlar. Call your doctor right away if you have signs of heart problems like a cough or shortness of breath that is new or worse, swelling of the ankles or legs, a heartbeat that does not feel normal, weight gain of more than 5 pounds in 24 hours, dizziness, or passing out.
What are some other side effects of Tafinlar?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Hair loss.
- Change in skin to hard and thick.
- Dry skin.
- Headache.
- Back, muscle, or joint pain.
- Constipation.
- Cough.
- Nose or throat irritation.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
Tafinlar Images
-
Tafinlar 75 mg
How do I store and/or throw out Tafinlar?
- Store at room temperature in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
Principle display panel
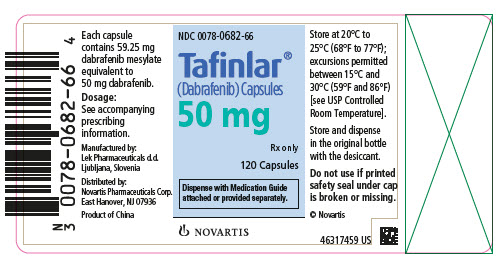
- NDC 0078-0682-66
- Tafinlar® (Dabrafenib) Capsules
- 50 mg
- Rx only
- 120 Capsules
- Dispense with Medication Guide attached or provided separately.
- NOVARTIS
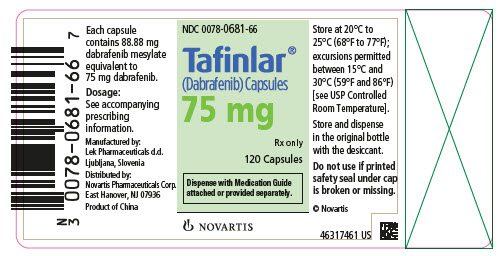
- NDC 0078-0681-66
- Tafinlar® (Dabrafenib) Capsules
- 75 mg
- Rx only
- 120 Capsules
- Dispense with Medication Guide attached or provided separately.
- NOVARTIS



An intriguing discussion is worth comment. There’s no doubt that that you ought
to write more on this subject matter, it might not be a taboo subject but usually folks don’t discuss these subjects.
To the next! Best wishes!!
My web-site :: boosted xt