Susvimo
Generic name: ranibizumab
Dosage forms: injection for intravitreal use via Susvimo ocular implant
Drug class: Anti-angiogenic ophthalmic agents
What is Susvimo?
Susvimo (ranibizumab injection) is a prescription medicine used to treat adults with Neovascular (wet) Age-related Macular Degeneration (AMD) who have responded to at least two injections of a Vascular Endothelial Growth Factor (VEGF) inhibitor in the gel-like part of the eye (intravitreal).
It is not known if Susvimo is safe and effective in children.
Description
Ranibizumab is a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment for intraocular use. Ranibizumab binds to and inhibits the biologic activity of human vascular endothelial growth factor-A (VEGF-A). Ranibizumab, which lacks an Fc region, has a molecular weight of approximately 48 kilodaltons and is produced by an E. coli expression system in a nutrient medium containing the antibiotic tetracycline. Tetracycline is not detectable in the final product.
SUSVIMO (ranibizumab injection) is supplied as a sterile, clear to slightly opalescent, colorless to pale brown solution for intravitreal use via the SUSVIMO implant. Each single-dose vial contains 10 mg of ranibizumab, histidine HCl (0.1 mg), polysorbate 20 (0.01 mg), sucrose (8.2 mg), and Water for Injection, in 0.1 mL of solution with a pH of 5.5. The SUSVIMO implant is designed to contain approximately 0.02 mL (2 mg) of ranibizumab solution when filled. SUSVIMO does not contain an antimicrobial preservative.
Mechanism of Action
Ranibizumab binds to the receptor binding site of multiple biologically active forms of VEGF-A, including VEGF110. VEGF-A has been shown to cause neovascularization and leakage in models of ocular angiogenesis and vascular occlusion and is thought to contribute to pathophysiology of neovascular AMD. The binding of ranibizumab to VEGF-A prevents the interaction of VEGF-A with its receptors (VEGFR1 and VEGFR2) on the surface of endothelial cells, reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation.
What is the most important information I should know about Susvimo?
Susvimo (ranibizumab injection) is delivered into the eye using the Susvimo implant. The Susvimo implant and the procedures to insert, fill, refill and remove the eye (ocular) implant can cause serious side effects including:
- an eye infection (endophthalmitis). Endophthalmitis is an infection of the eyeball that can cause permanent damage to your eye including blindness. Call your healthcare provider right away if you have increasing eye pain, vision loss, sensitivity to light, or redness in the white of the eye. Endophthalmitis requires urgent (same day) medical or surgical treatment.
- a missing layer on top of the white part of the eye (conjunctival erosion). Conjunctival erosion is an area that becomes missing (defect) in the layer (conjunctiva) that covers the white part of the eye which may result in exposure of the implant. Call your healthcare provider right away if you have a sudden feeling that something is in your eye, if you have eye discharge, or watering of the eye. Conjunctival erosion may require surgical treatment.
- an opening of the layer that covers the white part of the eye (conjunctival retraction). Conjunctival retraction is an opening or gaping in the layer (conjunctiva) that covers the white part of the eye which may cause the implant to be exposed. Call your healthcare provider right away if you have a sudden feeling that something is in your eye, if you have eye discharge, or watering of the eye. Conjunctival retraction may require surgical treatment.
See “What are the possible side effects of Susvimo?” for other serious side effects that may happen while in treatment with Susvimo.
To help prevent or keep these side effects from becoming more serious follow all post-procedure instructions your healthcare provider gives you. See “How will I use Susvimo?”.
Who should not use Susvimo?
Do not receive Susvimo if you:
- have an infection in or around your eye.
- have active swelling around your eye that may include pain and redness.
- are allergic to ranibizumab or any of the ingredients in Susvimo. See the end of this Medication Guide for a complete list of ingredients in Susvimo.
Talk to your healthcare provider before receiving this Susvimo if you have any of these conditions.
What should I tell my healthcare provider before using Susvimo?
Before receiving Susvimo, tell your healthcare provider about all of your medical conditions, including if you:
- are currently taking or have recently taken medicines that lower the chance of blood clots forming in the body such as warfarin, low or regular doses of aspirin, or nonsteroidal anti-inflammatory drugs (NSAID).
- are pregnant or plan to become pregnant. It is not known if Susvimo will harm your unborn baby. You should use birth control during your treatment with Susvimo and for 12 months after your last dose of Susvimo.
- are breastfeeding or plan to breastfeed. It is not known if Susvimo passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive Susvimo.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Susvimo?
- Susvimo is implanted through the white part of the eye (sclera) by your healthcare provider.
- Your healthcare provider will refill your implant device every 6 months (about every 24 weeks).
- If you miss a scheduled refill, call your healthcare provider as soon as possible to reschedule your refill. Your next refill should be given 6 months after your last refill.
Your healthcare provider will give you instructions to follow after the implant insertion, the refill procedure, and the implant removal. The instructions may include:
After the Implant Insertion:
- Positioning of your head
- Keep your head above shoulder level for the rest of the day.
- Sleep with your head on 3 or more pillows during the day and night after your implant insertion.
- How to care for your eye
- Do not remove the eye shield from your eye until you are told to by your healthcare provider. At bedtime, continue to wear an eye shield for at least 7 nights following the implant insertion.
- Take all post-operative eye medicines as your healthcare provider tells you to.
- Do not push on the eye, rub the eye, or touch the area of the eye where the implant is located (underneath the eyelid in the upper and outer part of your eye) for 30 days following the implant insertion.
- Do not participate in strenuous activities until 1 month after the implant insertion or after talking to your healthcare provider.
- Magnetic Resonance Imaging (MRI) Implant Card
- Get your implant card from your healthcare provider after receiving the implant and keep the card in a safe place for future reference. The implant card contains important information about your Susvimo implant.
- Show your current and future healthcare providers your implant card. This is important if you need to have an MRI. You may only receive an MRI under very specific conditions if you have the Susvimo implant. Your healthcare provider will review the information on the implant card and will let you know if you should receive an MRI.
After the Refill Procedure:
- Do not push on the eye, rub the eye, or touch the area of the eye where the implant is located (underneath the eyelid in the upper and outer part of your eye) for 7 days following the refill procedure.
- Take eye drops exactly as your healthcare provider tells you to take them.
After the Implant Removal:
- Keep your head above shoulder level for the rest of the day.
- Sleep with your head on 3 or more pillows if lying down during the day and night after implant removal.
- Wear an eye shield for at least 7 nights following the implant removal.
- Do not participate in strenuous activities until 14 days following the implant removal.
- Give all post-operative drops, as told by your healthcare provider.
These are not all the instructions you may receive from your healthcare provider. Following all post-procedure instructions may help prevent serious side effects or keep side effects from becoming more serious. See “What is the most important information I should know about Susvimo?”.
What should I avoid while using Susvimo?
- Do not drive or use machinery until the eye shield can be removed and you can see.
- Avoid rubbing your eye or touching the area of your eye where the implant is located as much as possible while the implant is in place. If you have to rub or touch your eye, wash your hands first.
What are the possible side effects of Susvimo?
See “What is the most important information I should know about Susvimo?”.
In addition to those side effects listed above, the Susvimo implant and the procedures to insert, fill, refill and remove the eye (ocular) implant can cause other serious side effects including:
- Tear and separation of layers of the retina (Rhegmatogenous retinal detachment). Rhegmatogenous retinal detachment is a tear and separation of one of the layers of the retina in the back of the eye that senses light. Call your healthcare provider or go to the emergency room right away if you see flashing lights, see a curtain or veil covering part of your vision, have a change in your vision, or a loss of vision. Rhegmatogenous retinal detachment requires surgical treatment.
- Implant movement (Implant dislocation): Tell your healthcare provider right away if you notice that the implant has moved out of place. This movement may require surgical treatment to correct.
- Bleeding (Vitreous hemorrhage): Vitreous hemorrhage is bleeding within the gel-like substance (vitreous) inside of your eye. Call your healthcare provider right away if you have an increase in moving spots or what looks like spider webs in your vision as you may need an additional eye surgery.
- Bump on top of the white layer of the eye (Conjunctival bleb): Conjunctival bleb is a small bulge in the layer (conjunctiva) that covers the white part of the eye where the implant is inserted. This may be due to leakage of fluid from the inside of the eye. Call your healthcare provider right away if you have a sudden feeling that something is in your eye (foreign body sensation), see a bulge over the white part of your eye, if you have eye discharge, or watering in the eye. You may need medical or surgical treatment.
- Temporary decrease in vision after the Susvimo procedure.
The most common side effects of Susvimo include:
- blood on the white of the eye
- eye pain
- redness in the white of the eye
- sensitivity to light
These are not all the possible side effects of Susvimo.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Genentech at 1-888-835-2555.
General information about the safe and effective use of Susvimo
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Susvimo that is written for health professionals.
What are the ingredients in Susvimo?
Active ingredient: ranibizumab
Inactive ingredients: histidine HCl, polysorbate 20, sucrose.
Instructions for use for Susvimo
Susvimo
(ranibizumab injection)
For Susvimo ocular implant use
- Initial Fill and Implant Procedure
- Implant Removal Procedure
Initial Fill and Implant Procedure
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.
Susvimo procedures should be performed by an ophthalmologist experienced in vitreoretinal surgery.
Refer to the Susvimo (ranibizumab injection) 100 mg/mL prescribing information for a complete list of indications, contraindications, warnings, precautions, and adverse events.
Contents
Introduction
| 3 | Device Description |
| 4 | Components |
| 8 | Intended Use/Indications for Use |
| 8 | Contraindications |
| 8 | Warnings |
| 9 | Precautions |
| 10 | How Supplied, Handling, and Storage |
Instructions for Use
| 11 | Introduction and Materials |
| 13 | Preparatory Procedures |
| 14 | Infusion Line Placement Procedure |
| 15 | Conjunctival Dissection Procedure |
| 17 | Syringe Preparation and Initial Implant Fill |
| 24 | Implant Insertion |
| 34 | Disposal and Post-insertion Procedures |
| 35 | Explanation of symbols on product or package labeling |
| 36 | Susvimo Implant Card |
Introduction
These instructions include only the procedure for filling and inserting the Susvimo implant. For more information, refer to the Susvimo prescribing information for the refill-exchange procedure and Susvimo Instructions for use for the implant removal procedure.
Device Description
Susvimo is an intraocular drug delivery system designed to be used specifically with Susvimo (ranibizumab injection) 100 mg/mL. The system consists of an intraocular implant along with ancillary devices used to fill, insert, and explant (if needed) the implant.
The implant is a refillable drug reservoir that is inserted into the eye through the pars plana. The implant is secured within the scleral incision, with the extrascleral flange that remains visible through the conjunctiva following insertion. Once filled with ranibizumab, the implant is designed to provide continuous release of ranibizumab. The implant will be refilled with ranibizumab in an office-based setting via an administration through the conjunctiva and implant septum.
4. Components
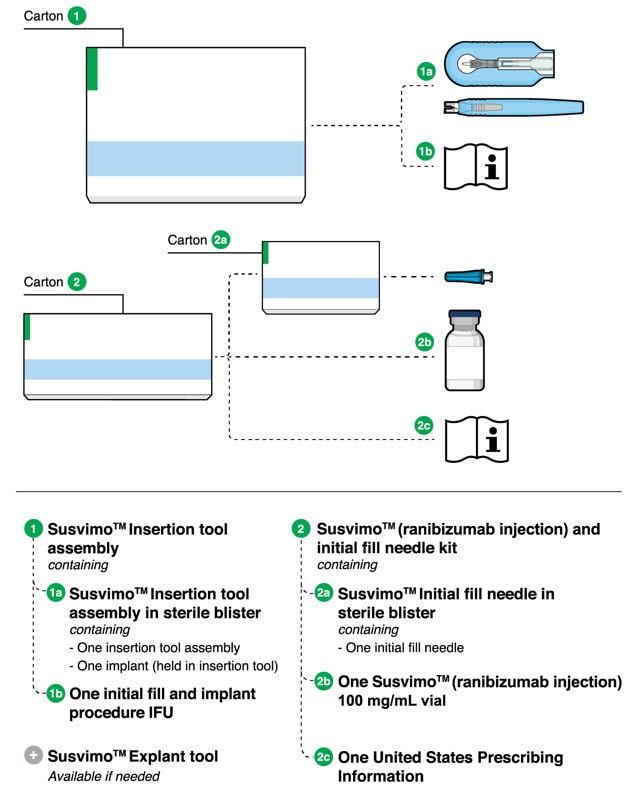
Figure 1.
Susvimo components for initial fill and implant procedure
Susvimo implant (packaged with insertion tool assembly)
Susvimo implant (Figure 2 and Figure 3) is a refillable reservoir inserted into the eye through the pars plana. The body of the implant, which includes the release control element, extends into the vitreous cavity.
Figure 2. Susvimo implant
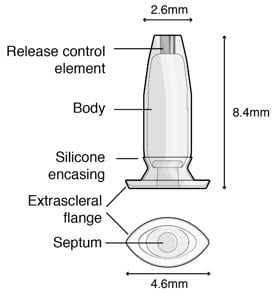
Figure 3. Susvimo implant detail
Table 1. Susvimo implant component description
| Implant components | Description |
| Extrascleral flange | The extrascleral flange provides secure anchoring of the implant within the scleral incision and is encased in silicone. |
| Septum | The septum is a self-sealing interface through which ranibizumab is administered into the implant both prior to insertion and during subsequent refills in an office-based setting. |
| Body | The body of the implant contains a hollow drug reservoir, capable of holding 0.02 mL of drug. |
| Release control element | The titanium release control element controls the rate of ranibizumab diffusion from the drug reservoir into the vitreous. |
Susvimo insertion tool assembly
Susvimo insertion tool assembly is designed to facilitate handling of the implant during the initial fill and implant procedure. The insertion tool assembly is comprised of a carrier and handle (Figure 4 and Figure 5), described in more detail below:
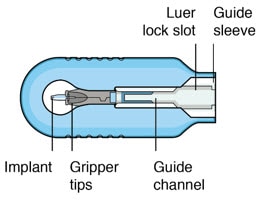
Figure 4. Susvimo insertion tool carrier
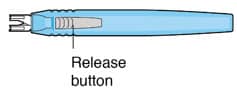
Figure 5. Susvimo insertion tool handle
Table 2. Susvimo insertion tool assembly component description
| Insertion tool assembly components | Description |
| Gripper tips | The implant is provided pre-positioned in the insertion tool assembly gripper tips. After initial fill of the implant with ranibizumab, the implant and gripper tips are transferred from the insertion tool carrier to the insertion tool handle. |
| Luer lock slot | The syringe is loaded into the insertion tool carrier by aligning the syringe Luer lock with the insertion tool carrier Luer lock slot. |
| Guide channel | The guide channel serves to direct the syringe and initial fill needle in the insertion tool carrier during the initial fill of the implant. |
| Release button | Pressing the release button on the insertion tool handle will open the gripper tips and release the implant. |
Susvimo (ranibizumab injection) 100 mg/mL vial
Susvimo (ranibizumab injection) (Figure 6) is used to fill the implant prior to insertion or during subsequent refill-exchange in an office-based setting.
Figure 6. Susvimo (ranibizumab injection) 100 mg/mL vial
Susvimo initial fill needle
Susvimo initial fill needle (Figure 7) is designed to fill the implant with ranibizumab prior to implant insertion. The initial fill needle is distinguished by its blue cap.
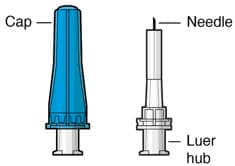
Figure 7. Susvimo initial fill needle
Table 3. Susvimo initial fill needle component description
| Initial fill needle components | Description |
| Needle | 34 G needle |
| Integrated filter | Integrated 5 µm filter within needle hub |
Intended Use/Indications for Use
Susvimo ocular implant is approved for use with Susvimo (ranibizumab injection). Refer to the Susvimo (ranibizumab injection) prescribing information for a complete list of indications, contraindications, warnings, precautions, and adverse events.
Contraindications
Susvimo is contraindicated in patients with ocular or periocular infections, with active intraocular inflammation, or with known hypersensitivity to ranibizumab or any of the excipients in Susvimo (ranibizumab injection) 100 mg/mL. Hypersensitivity reactions may manifest as severe intraocular inflammation.
Warnings
- Do not use if the sterility has been compromised or the contents have been dropped, damaged or tampered with.
- Minimize air bubbles within the implant reservoir as they may cause slower drug release. If an air bubble is present, it must be no larger than 1/3 of the widest diameter of the implant.
If excess air is observed after initial fill, do not use the implant. - Perpendicular entry of the implant is important to avoid contact between the implant and intraocular structures such as the lens, as contact between the implant and the intraocular structures may cause adverse events such as traumatic cataract.
- Avoid excessive force on the globe by first ensuring that the tip of the implant has passed through the sclero-pars plana incision before slowly pushing the implant into place.
Precautions
- Read and follow all instructions, warnings, and cautions prior to use.
- Susvimo procedures should be performed by an ophthalmologist experienced in vitreoretinal surgery.
- Use the Susvimo components and materials as specified in these instructions to perform the implant insertion procedure including initial fill.
- Avoid contact between sharp surgical instruments and the implant as the material of the septum and silicone encasing are soft and susceptible to damage.
- The implant is MR Conditional. The Patient Implant card is provided with instructions and must be completed and given to the patient after implant insertion. For further information please refer to the ‘Post-insertion patient instructions’ section.
Use with Standard Procedures
Susvimo implant is compatible for use with the following standard procedures: A-scan ophthalmic ultrasound, slit lamp examination, indirect ophthalmoscopy, tonometry, optical coherence tomography (OCT), visual field (perimetry), standard lasers for ophthalmic treatments, radiography (x-ray), computed tomography (CT) scan, fluorescein/ indocyanine angiography, and fundus autofluorescence.
Use caution when performing ophthalmic procedures that may cause deflection of the implant and subsequent injury. For example, B-scan ophthalmic ultrasound, scleral depression, or gonioscopy.
Magnetic Resonance Imaging (MRI) Safety Information
 MR Conditional.
MR Conditional.
Non-clinical testing has demonstrated that the Susvimo implant is MR Conditional. A patient with this device can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 1.5-Tesla (1.5 T) or 3-Tesla (3 T)
- Maximum spatial field gradient of 3,000 G/cm (30 T/m)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4.0 W/kg (First Level Controlled Operating Mode)
Under the scan conditions defined above, the Susvimo implant is expected to produce a maximum temperature rise of less than 1°C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the device extends approximately 4 mm from the Susvimo implant when imaged with a gradient echo pulse sequence in a 3 T MRI system.
How Supplied, Handling, and Storage
- All Susvimo components are supplied sterile.
Do not reprocess or resterilize. - All Susvimo components are for single use only.
Do not reuse Susvimo components. - Do not open sealed tray until time of use.
- Do not use if the package is damaged or broken as sterility may be compromised.
- Do not use past the expiration date printed on the label.
Susvimo ocular implant and insertion tool assembly
- The sealed tray has been sterilized with ethylene oxide gas.
- Store the Susvimo implant and insertion tool assembly at room temperature 15°C to 25°C (59°F to 77°F).
Susvimo (ranibizumab injection) and initial fill needle kit
- Susvimo initial fill kit should be stored at 2°C to 8°C (36°F to 46°F). Do not freeze. Protect from light. Do not shake. Prior to use, the unopened vial may be kept at 9°C to 30°C (48°F to 86°F) for up to 24 hours.
- Susvimo initial fill needle has been sterilized with electron beam processing.
See Susvimo (ranibizumab injection) prescribing information for additional information.
Instuctions for Use
Introduction and Materials
Implant insertion is a surgical procedure that is performed in an operating room. The implant is filled with Susvimo (ranibizumab injection) immediately prior to insertion.
Materials List
The materials that are required in the operating room on the day of the procedure are listed in Tables 4 and 5.
Table 4. Susvimo components provided for initial fill and implant procedure
| Item Description |
| Susvimo ocular implant with insertion tool assembly |
| Susvimo initial fill needle, 34 G, with blue cap |
| Susvimo (ranibizumab injection) 100 mg/mL |
| Susvimo explant tool (Refer to Susvimo implant removal Instructions for use for information on implant removal.) |
Table 5. Additional materials required but not provided for initial fill and implant procedure
| Item Description |
| One sterile 1 mL Luer Lock syringe (not included) |
| One sterile 5-micron filter needle (19-gauge × 1½ inch) (not included) |
| Surgical microscope |
| Vitrectomy surgical control system |
| Standard 25 G or 27 G vitrectomy set up |
| 23 G or 25 G 532 nm Endolaser probe and associated source |
| Standard vitrectomy tray (including adjustable caliper, 0.12 straight toothed forceps, blunt wescott scissors) |
| Cauterization equipment (including standard fine tip diathermy and eraser tip wet-field cautery) |
| Ophthalmic broad-spectrum microbicide solution |
| Marking pad |
| 3.5 mm and 4.0 mm fixed caliper or equivalent fixed tool |
| 3.5 mm fixed width gauge or equivalent fixed tool |
| 19 G or 20 G MVR Straight Knife |
| Slit Knife, 3.2 mm Straight |
| Gut or Vicryl sutures for conjunctival tissues (suggested 7-0 to 9-0: monofilament recommended) |
| Indirect ophthalmoscope and lens |
| Drapes |
Preparatory Procedures
1. Inspect packaging and components
- Prior to use in the operating room, inspect the packaging of the components for damage.
- Check the expiration date printed on the label.
- Remove the vial from the carton.
NOTE: the outside of the vial is not sterile. - Open sterile packaging and using aseptic technique, remove the components from their tray.
- Inspect components and place onto sterile field (Figure 8).
 Warning
Warning
Do not use if the sterility has been compromised or the contents have been dropped, damaged, or tampered with.
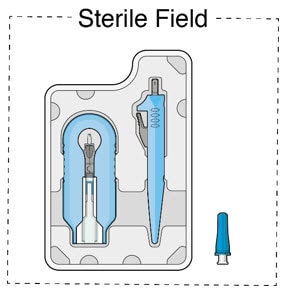
Figure 8. Susvimo components on sterile field
2. Inspect Susvimo (ranibizumab injection)
- Visually inspect the contents of the ranibizumab vial for particulate matter and discoloration.
- The drug solution should be colorless to pale brown.
 Caution
Caution
Do not use if particulate, cloudiness or discoloration are visible.
3. Patient Preparation
- Dilate the pupil of the eye.
- Place the patient in a supine position on the operating table.
- Implant insertion is a surgical procedure and therefore requires sterile controls (i.e. use of broad-spectrum microbicide solution on eye including lids and lashes and draping) be in place to minimize the risk of ocular infection.
- Perform the procedure under local anesthesia using either peribulbar, retrobulbar, or sub-Tenon’s technique.
- Place lid speculum.
Infusion Line Placement Procedure
1. Place infusion line
- Place an infusion cannula in the inferotemporal quadrant via an angled entry wound.
- Alternate placement is acceptable based on patient anatomy per physician discretion (superotemporal placement should be avoided).
- Alternatively, the line may be placed after the peritomy but prior to the scleral dissection.
- Attach the infusion line. Keep the infusion line off (Figure 9).
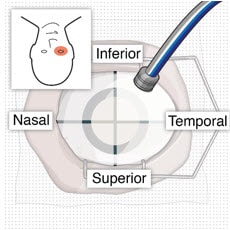
Figure 9. Place infusion cannula in inferotemporal quadrant
Conjunctival Dissection Procedure
1. Identify the site of insertion
- Identify the site in the superotemporal quadrant 4 mm posterior to the limbus where the implant will be inserted (Figure 10 and Figure 11).
- Placing a traction suture is strongly recommended for better visualization of the superotemporal quadrant throughout the entire implant insertion procedure.
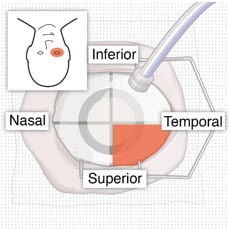
Figure 10. Superotemporal quadrant
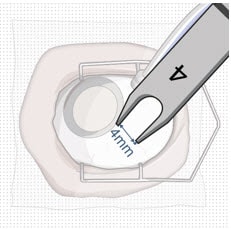
Figure 11. 4 mm posterior to the limbus
2. Create conjunctival peritomy
 Caution
Caution
The peritomy size should be at least 6 mm by 6 mm centered around the selected implant location to ensure the proper clearance of the conjunctiva and Tenon’s capsule from the implant flange once the implant is inserted into the eye.
- Measure with adjustable caliper and create at least a 6mm by 6mm peritomy of the conjunctiva and Tenon’s capsule around the selected implant location, using wet field cautery (eraser tip) to achieve hemostasis of the underlying episcleral tissues (Figure 12).
- A peritomy size of at least 6 mm by 6 mm centered around the selected implant location provides proper clearance of the conjunctiva and Tenon’s capsule for the implant flange (long axis of implant flange = 4.6 mm).
- A peritomy size of at least 6 mm by 6 mm facilitates implant placement away from radial relaxing incision.
- Appropriate peritomy size is vital for ease of subsequent surgery.
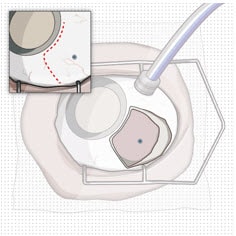
Figure 12. Conjunctival peritomy with blue dot representing selected implant location
- Only one radial incision is recommended, to avoid excessive conjunctival suturing and longer healing process.
- Careful incision creation is key to maintain integrity of conjunctiva and Tenon’s capsule. Careful and generous undermining is key to minimize mechanical tension and adequate coverage of the implant while closing the conjunctiva and Tenon’s capsule.
- Maintain hemostasis around the scleral incision throughout the surgery to facilitate the identification and management of incisional bleeding, to avoid post-operative vitreous hemorrhage.
Syringe Preparation and Initial Implant Fill
Using aseptic technique, the implant will be filled with 0.02 mL of ranibizumab prior to insertion of the implant into the patient’s eye.
1. Transfer dose from vial to syringe
 Caution
Caution
Use the filter needle to withdraw ranibizumab from the vial.
Do not use the initial fill needle for this step.
- Prepare ranibizumab vial by removing the flip-off cap and disinfecting the rubber vial septum with alcohol.
- Attach a filter needle to the syringe by screwing it tightly onto the Luer lock (Figure 13).
- Carefully remove the needle cap by pulling it straight off.
- Using aseptic technique, withdraw all of the contents of the ranibizumab vial through the filter needle into the syringe.
- Remove and properly dispose of the filter needle after withdrawal of the vial contents.
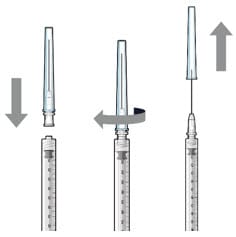
Figure 13. Filter needle attached to syringe and cap removal
2. Attach initial fill needle
- Attach the initial fill needle firmly onto the syringe by screwing it tightly onto the Luer lock (Figure 14).
- Carefully remove the needle cap by pulling straight off.
- Do not wipe the needle at any time.
 Caution
Caution
Ensure that the initial fill needle is attached to the syringe.
Do not use the filter needle to fill the implant.
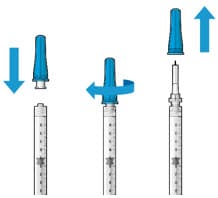
Figure 14. Initial fill needle attached to syringe and cap removal
3. Remove air from syringe
- Hold the syringe with the needle pointing up.
- If there are any air bubbles, gently tap the syringe with your finger until the bubbles rise to the top (Figure 15).
- Slowly push the plunger rod just until all air is expelled from the syringe and needle, and a drop of drug solution is seen at the needle tip (Figure 16).
- It is important to preserve as much drug as possible in order to completely fill the implant.
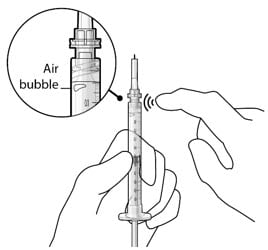
Figure 15. Gently tap the syringe to dislodge bubbles
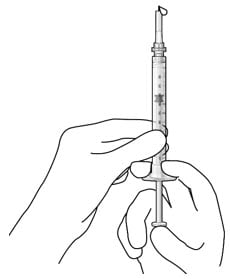
Figure 16. No air bubbles and a drop of drug at the needle tip
4. Inspect the syringe for air bubbles
- Inspect the syringe and the needle hub to ensure that no air bubbles are present (Figure 17).
- If air bubbles are present, continue to remove air from the syringe and reinspect.
 Caution
Caution
Use the syringe within 15 minutes of removing all air to avoid ranibizumab drying in the needle and impeding fluid flow.
Do not use the initial fill needle if the needle is clogged.
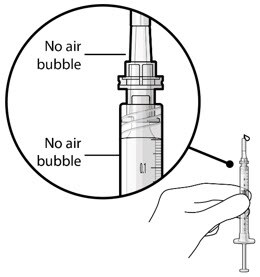
Figure 17. No air bubbles in the syringe and needle hub
5. Load syringe into the carrier
 Caution
Caution
Do not hold or push on the plunger rod of the syringe while inserting the needle into the implant septum.
- Retrieve insertion tool carrier with pre-positioned implant from the inner tray.
- Align the syringe Luer lock above the Luer lock slot in the carrier to protect the needle from being damaged.
- Lower the syringe into the carrier (Figure 18).
- Push the syringe forward until it stops, taking care to avoid touching the plunger rod (Figure 19).
- With the syringe loaded, (Figure 20) the initial fill needle should now be penetrating the implant septum.
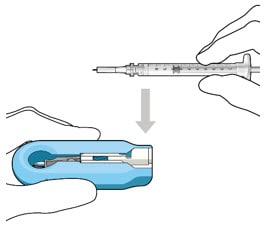
Figure 18. Align and lower the syringe into the carrier
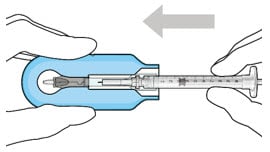
Figure 19. Push the syringe into the carrier
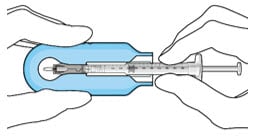
Figure 20. Syringe with initial fill needle inserted through the implant septum
6. Fill implant with ranibizumab under microscope
- Under the microscope, slowly administer ranibizumab into the implant by slightly tilting the carrier upwards (Figure 21).
- The implant should be filled over approximately 5 to 10 seconds, to help avoid air entrapment in the implant reservoir.
- Continue filling the implant until the implant is completely full of drug solution and all air has been expelled as evidenced by a dome of drug solution formed at the tip of the implant on the release control element (Figure 22).
 Caution
Caution
When filling the implant, fluid should only exit the implant from the release control element. If fluid is leaking from the implant at a different location, such as the side of the implant, do not use the implant.
If fluid is leaking from the septum at the needle insertion site, the needle may not be fully penetrating the implant septum. Fully push the syringe forward before continuing to fill the implant.
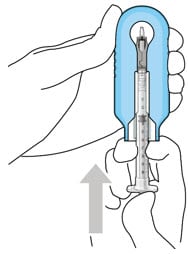
Figure 21. Administer ranibizumab into the implant
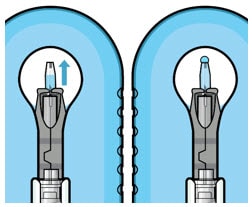
Figure 22. Dome of drug solution forms at tip of implant as viewed under magnification
7. Inspect the filled implant under the microscope
- Inspect the implant under the microscope to ensure that the implant is completely full of drug solution (Figure 23).
 Warning
Warning
Minimize air bubbles within the implant reservoir as they may cause slower drug release. If an air bubble is present, it must be no larger than 1/3 of the widest diameter of the implant.
If excess air is observed, do not use the implant. Caution
Caution
No more than 30 minutes should pass between the initial fill of the implant and the insertion into the patient’s eye to ensure that the release control element remains saturated with ranibizumab. If ranibizumab dries in the release control element, the implant may not release the drug properly into the vitreous after insertion.
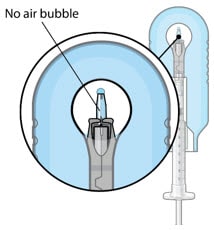
Figure 23. Proper appearance of implant after initial filling with ranibizumab
8. Remove the syringe and guide sleeve from the carrier
- Remove the syringe and guide sleeve from the carrier by pulling back on the syringe (Figure 24). The syringe will be locked into the guide sleeve.
- Properly dispose of the used syringe together with the needle and guide sleeve in a sharps disposal container or in accordance with local requirements.
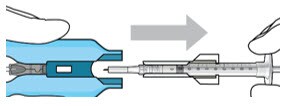
Figure 24. Remove the syringe and guide sleeve from the insertion tool carrier
9. Slide the insertion tool handle into the carrier
- Slide the insertion tool handle into the guide channel of the carrier, ensuring that both components are facing upwards (Figure 25).
- Push the handle forward as far as it will go into the gripper tips (Figure 26).
 Caution
Caution
Do not withdraw the handle and implant until the eye is ready for insertion. Contact between the implant and any surface or object – even within the sterile field – may result in the introduction of a foreign body into the vitreous.
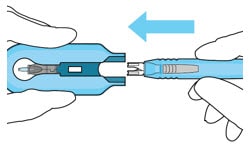
Figure 25. Insert the handle into the insertion tool carrier
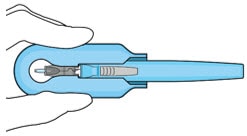
Figure 26. Fully inserted handle
Implant Insertion
1. Verify hemostasis of the scleral surface
- Clear any excess blood from the scleral surface.
- Perform careful scleral wet field cauterization as needed, particularly in the area of the sclero-pars plana incision.
2. Mark incision site
- Keep sclera dry and create discrete ink marks using a light touch.
- Mark a location 4 mm from the limbus using an inked fixed width caliper (or equivalent) at the selected implant location (Figure 27).
- Mark a 3.5 mm length for scleral dissection parallel to and 4 mm posterior to the limbus using an inked fixed width caliper (or equivalent) (Figure 28).
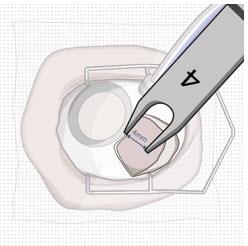
Figure 27. 4 mm posterior to the limbus
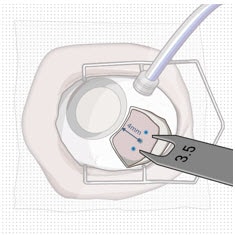
Figure 28. Fixed width caliper measurement
3. Perform scleral incision
- Using an MVR blade, create a full thickness dissection of the sclera until the pars plana is fully visible (Figure 29).
- Ensure scleral dissection location is away from the radial conjunctival incision.
 Caution
Caution
The final target length for the scleral incision is 3.5 mm. Keep in mind that laser may further result in enlargement of incision and limit the secureness of the fit of the implant.
- Confirm the length of the incision by using the largest width of the 3.5 mm incision gauge (Figure 30).
- If the incision is over 3.5 mm, as indicated by a loose fit/side to side motion of 3.5 mm gauge, place a suture through the wound opposite the relaxing incision to reduce the incision down to 3.5 mm, and bury the knot. After suturing, confirm the incision length is 3.5 mm (Figure 30).
- If there are any areas of visible bleeding from the wound, carefully perform diathermy.
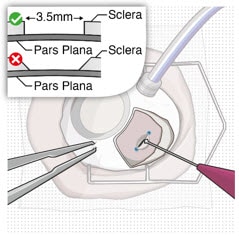
Figure 29. Stabilize the globe and perform full thickness scleral incision
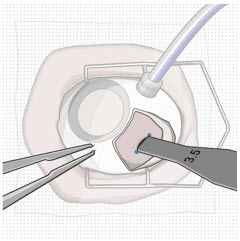
Figure 30. Confirm correct length of scleral incision
4. Perform laser treatment of the pars plana
 Caution
Caution
Laser treatment may result in enlargement of the incision. Laser treatment should not be used to intentionally enlarge the length of scleral incision.
 Caution
Caution
Carefully apply the laser only to the choroidal tissue in the exposed pars plana. Minimize any laser application to the surrounding scleral tissue to avoid damaging tissue integrity.
- Confirm pars plana is dry before laser treatment and keep dry throughout the procedure (with surgical sponge).
- Consider placing a surgical sponge as a wick at one end of the incision.
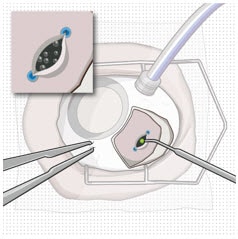
Figure 31. Laser treatment of the pars plana
- Using a 532 nm laser endoprobe, apply contiguous, overlapping laser spots starting at 300 mW 1000 ms along the full length of the exposed pars plana (Figure 31). Repeat until complete ablation is achieved.
- Maintain focus of laser on exposed pars plana.
- Keep foot pedal depressed to achieve full 1000 ms spots (expect smoke).
- Ensure the pars plana at the corners of scleral incision is adequately treated.
- Do not use painting strokes with the laser.
- Do not contact the pars plana directly with laser probe.
- Repeat application of laser along the full length of the pars plana until full or partial split of the pars plana, or other visual endpoints are achieved, as indicated by:
- Gray color change.
- Uniform perforated appearance.
- Domes of vitreous fluid percolating through pars plana.
- If visual endpoint is not achieved after several passes with the laser, increase laser power in 100 mW increments.
- Once laser ablation of pars plana is completed, remeasure the entire length (including corners) of the scleral incision with 3.5 mm gauge to confirm the final post-laser incision is 3.5 mm. If final incision is greater than 3.5 mm, as indicated by a loose fit/side to side motion of 3.5 mm gauge, place a suture through the wound opposite the relaxing incision to reduce incision down to 3.5 mm.
 Caution
Caution
A final post-laser incision length of 3.5 mm provides a secure fit for the implant.
Do not enlarge the scleral dissection beyond 3.5 mm as a final incision length greater than 3.5 mm may result in an improperly seated implant and will require additional suturing.
5. Perform pars plana incision
- Pass a 3.2 mm slit knife perpendicularly through the center of the scleral incision to open the underlying pars plana (Figure 32 and Figure 33).
- Ensure widest part of the blade passes through the incision.
- Ensure adequate hemostasis.
- Carefully check for any active bleeding from the pars plana incision; if active bleeding is present, address it with fine tip diathermy within the incision before proceeding.
 Caution
Caution
Do not enlarge the pars plana incision with lateral movements. The incision width of 3.2 mm ensures that the pars plana incision is within the laser-treated area of the pars plana and reduces the risk of vitreous hemorrhage.
- Aim to the center of the globe.
- Insert blade straight in and straight out.
- Avoid sideways motion.
- Avoid enlargement of the incision.
- Avoid the edges of the wound.
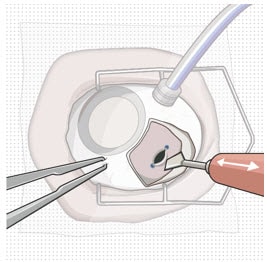
Figure 32. Pars plana incision
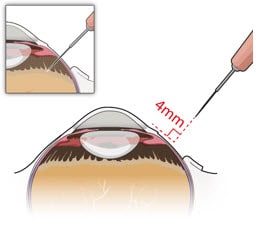
Figure 33. Perpendicular entry of slit knife
6. Withdraw the insertion tool handle with filled implant
- Withdraw the insertion tool handle, which is now attached to the gripper tips and implant, by slowly pulling the carrier and handle apart (Figure 34).
- Take care not to touch implant to any surface other than incision.
 Caution
Caution
When holding the insertion tool handle, take care not to touch the implant to any surface other than the sclero-pars plana incision during the insertion procedure.
Do not use the implant if it is inadvertently released or is contaminated through contact with any surface other than the exposed sclera, including objects within the surgical field.
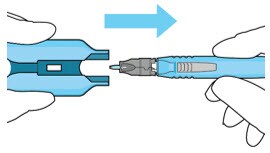
Figure 34. Withdraw the insertion tool handle with filled implant
7. Stabilize the globe
- Prior to inserting the implant, stabilize the globe with forceps to prevent unwanted eye movement (Figure 35).
- The forceps should remain in place until the insertion procedure is complete.
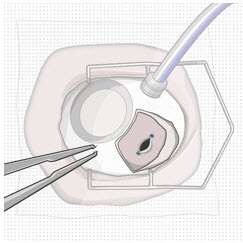
Figure 35. Stabilize the globe with forceps
8. Orient the implant
- Orient the long axis of the implant flange with the length of the sclero-pars plana incision (Figure 36).
- Aligning the implant in this direction enables the intended seating of the implant within the sclero-pars plana incision.
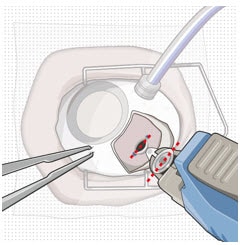
Figure 36. The long axis of the implant aligned with the length of the sclero-pars plana incision
9. Insert the implant
 Warning
Warning
Perpendicular entry of the implant is important to avoid contact between the implant and intraocular structures such as the lens, as contact between the implant and the intraocular structures may cause adverse events such as traumatic cataract.
 Warning
Warning
Avoid excessive force on the globe by first ensuring that the tip of the implant has passed through the sclero-pars plana incision before slowly pushing the implant into place.
- Slowly insert the implant through the sclero-pars plana incision perpendicular to the globe (Figure 37 and Figure 38).
- A slight initial twisting motion may be helpful to ease the implant through the sclero-pars plana incision (Figure 39).
- Continue pressing the implant slowly through the incision until the insertion tool handle gripper tips abut the sclera.
- Per the surgeon’s discretion, the infusion line may be turned on while inserting the implant.
- If excess vitreous prolapses from the incision, use the vitrector to remove it and then place the implant.
- If a small amount of vitreous prolapse is present, place the implant first and then use the vitrector to remove the excess. Only use the vitrector to remove vitreous prolapse (do not use surgical sponge and/or scissors).
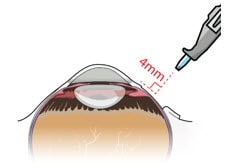
Figure 37. Perpendicular entry of the implant
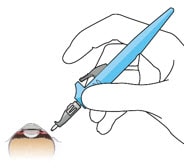
Figure 38. Implant insertion
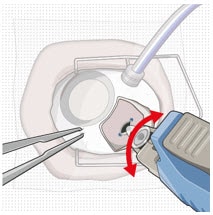
Figure 39. Implant insertion with a slight initial twisting motion
10. Release the implant
- Ensure that the long axis of the implant is properly aligned with the sclero-pars plana incision before releasing the implant from the insertion tool.
- The forceps that are stabilizing the globe should remain in place.
- Release the implant by depressing the release button completely (Figure 40 and Figure 41).
 Caution
Caution
If the implant flange is not parallel to the limbus after releasing, it needs to be repositioned solely using the gripper tips of the insertion tool handle. Reposition gently to avoid damage to the implant and to avoid contact between the implant and intraocular structures such as the lens. Avoid excessive manipulation of the implant flange.
Do not use any other rigid instruments to reposition.
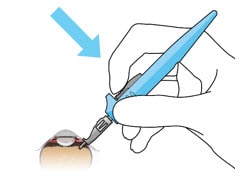
Figure 40. Release implant with the release button
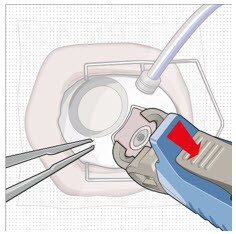
Figure 41. Implant release
11. Seat the implant
 Caution
Caution
Use the insertion tool gripper tips to seat the implant.
Do not use any other rigid instruments as they may damage the implant.
 Caution
Caution
Ensure that the long axis of the implant is aligned with the length of the sclero-pars plana incision and that the implant is seated flush against the sclera.
- Remove finger from the release button to close the insertion tool gripper tips.
- Gently press the closed gripper tips against the center of the implant to seat the implant flush against the sclera (Figure 42).
- Clean any residual vitreous around the implant flange using a vitrector.
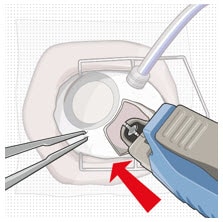
Figure 42. Seat the implant
12. Suture Tenon’s capsule and conjunctiva
- Close both Tenon’s capsule and conjunctiva, with Vicryl or gut sutures, ensuring complete coverage of the implant flange.
- Use scleral anchoring at the apex of the peritomy.
- Ensure suture placement away from the implant (Figure 43).
 Caution
Caution
Do not place a suture directly over the implant, otherwise adverse events including incomplete healing, infection, and discomfort can occur.
 Caution
Caution
Complete closure of both Tenon’s capsule and conjunctiva across the surgical site is critical to minimize potential complications such as conjunctival retraction over the implant.
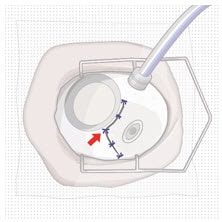
Figure 43. Suture conjunctiva
13. Remove infusion cannula
- If the infusion was previously turned on, then set the infusion pressure to 20 mmHg before removing the infusion cannula.
- Check for persistent leaks at the infusion cannula site and suture if necessary.
14. Check Intraocular Pressure (IOP)
- Using digital palpation, check the IOP. If necessary, inject additional fluid to restore IOP.
15. Check implant placement
- Perform indirect ophthalmoscopy to confirm implant position and to examine for the presence of any complications.
Disposal and Post-insertion Procedures
1. Dispose of used Susvimo components and tools
- Do not recap the needle or detach it from the syringe. Dispose of the used Susvimo components and tools in a sharps disposal container or in accordance with local requirements.
2. Perform post-insertion procedures
- Post-insertion procedures are consistent with standard post-surgical procedures.
3. Post-insertion patient instructions
Provide the patient with the following post-operative instructions:
- Positioning:
- Keep head above shoulder level for the rest of the day.
- Sleep with head elevated on 3 or more pillows if lying down during the day and night after implant insertion.
- Information on caring for the eye after the procedure, including but not limited to the following:
- Do not remove the eye shield until they are instructed to do so by their physician. At bedtime, continue to wear the eye shield for at least 7 nights following implant insertion.
- Administer all post-operative eye medications, as directed by their physician.
- Do not push on the eye, rub the eye, or touch the region of the eye where the implant is located (underneath the eyelid in the upper and outer part of the eye) for 30 days following implant insertion. Avoid rubbing the eye or touching the area where the implant is located as much as possible at all other times but if necessary to do so, make sure hands are cleaned prior to touching the eye.
- Do not participate in strenuous activities until 1 month after implant insertion or after discussion with their physician.
- Monitor for symptoms that may require immediate medical attention while the implant is in place.
- MR Conditional information:
- The surgeon should inform the patient that the implant is MR Conditional (as noted on their Susvimo implant card) and if patient needs to undergo an MRI, they should let their doctor know they have Susvimo implanted in their eye.
- After implant insertion, the surgeon should give the patient the implant card (enclosed in this IFU on the last page) with the appropriate information filled in, and should advise the patient to keep the card in a safe place, e.g. his or her wallet, for future reference. The surgeon should advise the patient that this implant card contains important information related to the Susvimo implant and that the card should be shown to their current and future health care providers.
Explanation of symbols on product or package labeling
Table 6. Symbols on blister tray and carton
| Symbol and Title |
 Manufacturer Manufacturer |
 Prescription only Prescription only |
 Do not re-use Do not re-use |
 Do not use if package is damaged Do not use if package is damaged |
 Consult Instructions for Use Consult Instructions for Use |
 Sterilized by irradiation Sterilized by irradiation |
 Sterilized using ethylene oxide Sterilized using ethylene oxide |
 Temperature limit Temperature limit |
 Expiration date/Use by date Expiration date/Use by date |
 Lot/Batch number Lot/Batch number |
 MR Conditional MR Conditional |

Note – The Implant LOT number can be found on the carton and blister tray of the Susvimo Insertion Tool Assembly
Instructions for use approved 10/2021
Implant Removal Procedure
Caution: Federal Law (USA) restricts this device to sale by or on the order of a physician.
Susvimo procedures should be performed by an ophthalmologist experienced in vitreoretinal surgery.
Refer to the Susvimo (ranibizumab injection) 100 mg/mL prescribing information for a complete list of indications, contraindications, warnings, precautions, and adverse events.
Contents
Introduction
| 3 | Device Description |
| 3 | Components |
| 4 | Intended Use/ Indications for Use |
| 4 | Warnings |
| 4 | Precautions |
| 5 | How Supplied, Handling, and Storage |
Instructions for Use
| 6. | Introduction and Materials |
| 7. | Preparatory Procedures |
| 8. | Implant Removal |
| 16 | Disposal and Post-removal Procedures |
| 17 | Explanation of symbols on product or package labeling |
Introduction
These instructions include only the implant removal procedure for the Susvimo implant. Refer to the Susvimo Instructions for use for the initial fill and implant procedure and prescribing information for the refill-exchange procedure.
Device Description
Susvimo is an intraocular drug delivery system designed to be used specifically with Susvimo (ranibizumab injection) 100 mg/mL.
These Instructions for Use describe the required materials and steps necessary for removal of the Susvimo implant.
Components
Figure 1. Susvimo components for implant removal procedure
Susvimo explant tool
Susvimo explant tool (Figure 2) is a pair of forceps with contoured tips, which are designed to grasp underneath the implant flange to securely engage the implant during removal.
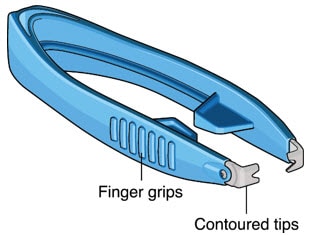
Figure 2. Susvimo explant tool
Intended Use/ Indications for Use
Susvimo explant tool is approved for use with Susvimo ocular implant. Refer to the Susvimo (ranibizumab injection) prescribing information for a complete list of indications, contraindications, warnings, precautions, and adverse events.
Warnings
- Do not use if the sterility has been compromised or the contents have been dropped, damaged or tampered with.
- Do not grasp the implant by the short axis of the implant flange. Remove the implant in a gentle manner. Perpendicular exit of the implant is important to avoid contact between the implant and intraocular structures such as the lens.
Precautions
- Read and follow all instructions, warnings, and cautions prior to use.
- Susvimo procedures should be performed by an ophthalmologist experienced in vitreoretinal surgery.
- Use Susvimo components and materials as specified in these instructions to perform the implant removal procedure.
How Supplied, Handling, and Storage
- Susvimo explant tool has been electron beam sterilized. Do not reprocess or resterilize.
- Susvimo explant tool is for single-use only. Do not reuse the Susvimo explant tool.
- Do not open sealed tray until time of use.
- Do not use if the package is damaged or broken as sterility may be compromised.
- Do not use past the expiration date printed on the label.
- Susvimo explant tool should be stored at a room temperature 15°C to 25°C (59°F to 77°F).
Instructions for Use
Introduction and Materials
Removal of the Susvimo implant requires a surgical procedure that is performed in an operating room.
Materials List
Materials that are required and provided for the procedure are listed in table 1.
Table 1. Susvimo sterile components that are provided for implant removal
| Item Description |
| Susvimo explant tool |
Materials required but not provided are listed Table 2.
Table 2. Additional materials required but not provided for implant removal
| Item Description |
| Surgical microscope |
| Vitrectomy surgical control system |
| Standard 25 G or 27 G vitrectomy set up |
| Standard vitrectomy tray (including 0.12 straight toothed forceps and blunt wescott scissors) |
| Cauterization equipment (including standard fine tip diathermy and eraser tip wet-field cautery) |
| Ophthalmic broad-spectrum microbicide solution |
| Surgical scalpel blade #15 |
| Non-absorbable sutures for sclera (suggested Nylon) |
| Gut or Vicryl sutures for conjunctival tissues (suggested 7-0 to 9-0: monofilament recommended) |
| Indirect ophthalmoscope and lens |
| Drapes |
Preparatory Procedures
1. Inspect and open sterile packaging
- Prior to use in the operating room, inspect the sterile packaging of the explant tool for damage.
- Check the expiration date printed on the label.
- Open sterile packaging and using aseptic technique, remove the explant tool from the tray.
- Inspect the explant tool and place onto sterile surface.
 Warning
Warning
Do not use if the sterility has been compromised or the contents have been dropped, damaged, or tampered with.
2. Patient Preparation
- Dilate the pupil of the eye.
- Place the patient in a supine position on the operating table.
- Implant removal is a surgical procedure and therefore requires sterile controls (i.e. use of ophthalmic broad-spectrum microbicide solution on eye including lids and lashes and draping) be in place to minimize the risk of ocular infection.
- Perform the procedure under local anesthesia using either peribulbar, retrobulbar, or sub-Tenon’s technique.
- Place lid speculum.
Implant Removal
Perform the implant removal procedure under the microscope. Consider placing a traction suture for better visualization of the superotemporal quadrant throughout the entire implant removal procedure.
1. Place infusion line
- Place an infusion cannula in the inferotemporal quadrant via an angled entry wound.
- Attach the infusion line, however keep the infusion off.
2. Perform conjunctival peritomy
- Create at least a 6 mm by 6 mm peritomy of the conjunctiva and Tenon’s capsule around the implant flange (Figure 3).
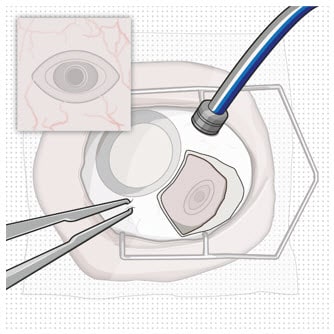
Figure 3. Conjunctival peritomy
3. Remove fibrous capsule covering implant
- Remove any fibrous capsule or scar tissue that may have formed over the implant flange and septum (Figure 4 and Figure 5).
- To expose the implant, use a scalpel to make an incision in the tissue around the entire circumference of the implant (Figure 4).
- Use forceps to carefully dissect the implant flange and neck from the surrounding tissue so that the edges of the flange are completely exposed and the implant is free of any adherent tissue (Figure 5).
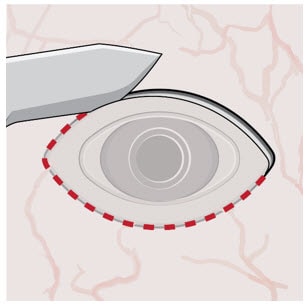
Figure 4. Incision to remove tissue that may be covering the implant flange and septum
Figure 5. Exposed implant
4. Stabilize the globe and align the explant tool
- Stabilize the globe with forceps to prevent unwanted eye movement.
- With the explant tool oriented perpendicular to the globe, align the contoured tips with the long axis of the implant flange (Figure 6).
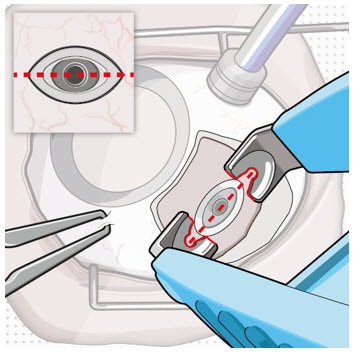
Figure 6. Align the explant tool along the long axis of the implant flange
5. Grasp the implant
 Warning
Warning
Do not grasp the implant by the short axis of the implant flange. Remove the implant in a gentle manner. Perpendicular exit of the implant is important to avoid contact between the implant and intraocular structures such as the lens.
- Use the contoured tips of the explant tool to gently grasp underneath the long axis of the implant flange (Figure 7).
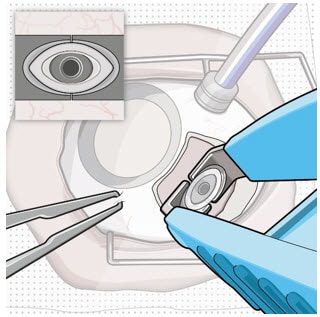
Figure 7. Grasp underneath the long axis of the implant flange
6. Remove the implant
- Once the implant is secured in the explant tool, in a gentle manner pull the implant from the eye in a perpendicular motion to avoid contact with adjacent intraocular structures.
- During removal, use forceps to stabilize the globe and provide counter traction to help release the surrounding tissue (Figure 8).
- During removal, continue to maintain perpendicular orientation of the implant relative to the globe (Figure 9).
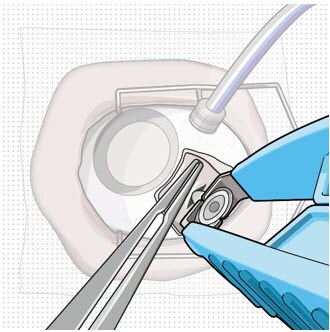
Figure 8. Implant removal
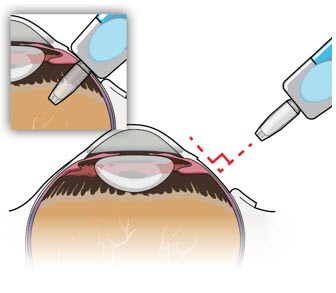
Figure 9. Perpendicular exit of the implant
- If needed per the surgeon’s discretion, the infusion line on the vitrectomy surgical system can be turned on during implant removal.
- Control any scleral or conjunctival bleeding, using cauterization if needed, and clear any vitreous prolapse present within or around the scleral wound using a vitrectomy setup.
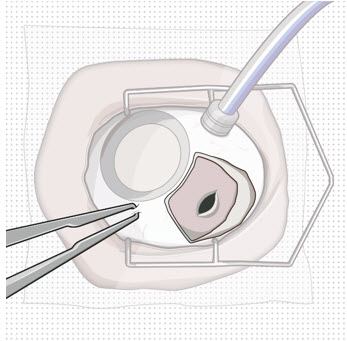
Figure 10. Appearance of the scleral wound after implant removal
7. Suture the sclera
- Completely close the scleral incision with non-absorbable (suggested Nylon) sutures (Figure 11). More than one suture will be needed to close the wound. It is recommended to place equally spaced partial thickness interrupted sutures.
- Ensure that the knots are buried.
 Caution
Caution
Do not overtighten the scleral sutures, to minimize excessive tensions on the sclera or cornea.
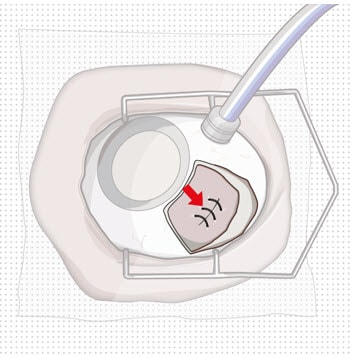
Figure 11. Suture scleral wound using multiple sutures
8. Suture conjunctiva
- Close Tenon’s capsule and conjunctiva to completely cover the scleral incision (Figure 12).
 Caution
Caution
Completely close Tenon’s capsule and conjunctiva to minimize potential post-removal complications.
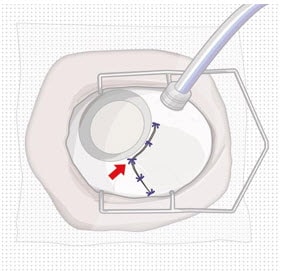
Figure 12. Suture conjunctiva post-removal
9. Remove infusion cannula
- If the infusion was previously turned on, then set the infusion pressure to 20 mmHg before removing the infusion cannula.
- Place suture at infusion cannula site as needed.
10. Check Intraocular Pressure (IOP) using digital palpation
11. Perform indirect ophthalmoscopy to examine for the presence of any complications
Disposal and Post-removal Procedures
1. Dispose of the used components
- Dispose of the used explant tool together with the implant in a biohazard waste container or in accordance with local requirements.
2. Perform post-removal procedures
- Post-removal procedures are consistent with standard post-surgical procedures.
3. Post-removal patient instructions
- Provide the patient with the following post-operative instructions:
- Positioning:
- Keep head above shoulder level for the rest of the day.
- Sleep with head elevated on 3 or more pillows if lying down during the day and night after implant removal.
- Information on caring for the eye after the procedure, including but not limited to:
- Monitor for symptoms that may require immediate attention.
- Wear an eye shield at bedtime for at least 7 nights.
- Do not participate in strenuous activities until 14 days following the implant removal procedure.
- Administer all post-operative anti-inflammatory and antimicrobial drops, as directed by their physician.
- Positioning:
Explanation of symbols on product or package labeling
Table 3. Symbols on blister tray and carton
| Symbol and Title |
 Manufacturer Manufacturer |
 Prescription only Prescription only |
 Do not re-use Do not re-use |
 Do not use if package is damaged Do not use if package is damaged |
 Consult Instructions for Use Consult Instructions for Use |
 Sterilized by irradiation Sterilized by irradiation |
 Sterilized using ethylene oxide Sterilized using ethylene oxide |
 Temperature limit Temperature limit |
 Expiration date/Use by date Expiration date/Use by date |
 Lot/Batch number Lot/Batch number |
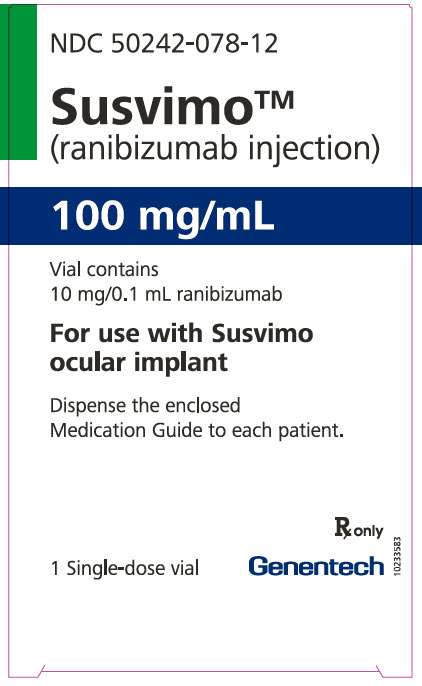
Label
PRINCIPAL DISPLAY PANEL – 10 MG/0.1 ML VIAL CARTON
- NDC 50242-078-12
- Susvimo™
(ranibizumab injection) - 100 mg/mL
- Vial contains
10 mg/0.1 mL ranibizumab - For use with Susvimo
ocular implant - Dispense the enclosed
Medication Guide to each patient. - Rx only
- 1 Single-dose vial
- Genentech
- 10233583