Ranibizumab
Generic name: ranibizumab (ophthalmic)
Brand names: Lucentis, Susvimo Implant Kit, Susvimo
Dosage form: intravitreal solution (10 mg/mL; 6 mg/mL)
Drug class: Anti-angiogenic ophthalmic agents
Medically reviewed by A Ras MD.
What is ranibizumab used for?
Ranibizumab is a prescription medicine that is used to treat macular degeneration. It is used to treat macular swelling. Ranibizumab is also used to treat some eye problems caused by diabetes.
Ranibizumab may be given to you for other reasons.
Description
BYOOVIZ (ranibizumab-nuna) is a recombinant humanized IgG1 kappa isotype monoclonal antibody fragment designed for intraocular use. Ranibizumab-nuna binds to and inhibits the biologic activity of human vascular endothelial growth factor A (VEGF-A). Ranibizumab-nuna, which lacks an Fc region, has a molecular weight of approximately 48 kilodaltons and is produced by an E. coli expression system in a nutrient medium containing the antibiotic tetracycline. Tetracycline is not detectable in the final product.
BYOOVIZ (ranibizumab-nuna) injection is a sterile, clear to slightly opalescent and colorless to pale yellow solution in a single-dose glass vial for intravitreal use. BYOOVIZ is supplied as a preservative-free, sterile solution in a single-dose container designed to deliver 0.05 mL of 10 mg/mL BYOOVIZ (0.5 mg dose vial) aqueous solution with 10 mM histidine HCl, 10% α,α-trehalose dihydrate, 0.01% polysorbate 20, pH 5.5.
Mechanism of Action
Ranibizumab products bind to the receptor binding site of active forms of VEGF-A, including the biologically active, cleaved form of this molecule, VEGF110. VEGF-A has been shown to cause neovascularization and leakage in models of ocular angiogenesis and vascular occlusion and is thought to contribute to pathophysiology of neovascular AMD, mCNV, and macular edema following RVO. The binding of ranibizumab products to VEGF-A prevents the interaction of VEGF-A with its receptors (VEGFR1 and VEGFR2) on the surface of endothelial cells, reducing endothelial cell proliferation, vascular leakage, and new blood vessel formation.
Before taking ranibizumab, tell your doctor:
- If you are allergic to ranibizumab; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have any kind of eye infection.
This is not a list of all drugs or health problems that interact with ranibizumab.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take ranibizumab with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take ranibizumab?
- Tell all of your health care providers that you take ranibizumab. This includes your doctors, nurses, pharmacists, and dentists.
- Use care when driving or doing other tasks that call for clear eyesight.
- Have your eye pressure and eyesight checked as you have been told by the doctor.
- This medicine may affect fertility. Fertility problems may lead to not being able to get pregnant or father a child.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using ranibizumab while you are pregnant.
- Tell your doctor if you are breast-feeding. You will need to talk about any risks to your baby.
How is ranibizumab best taken?
Use ranibizumab as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Your doctor will give ranibizumab.
- It is given as a shot into the eye.
What do I do if I miss a dose?
- Call your doctor to find out what to do.
What are the side effects of ranibizumab that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Change in eyesight, eye pain, or very bad eye irritation.
- Eyelid swelling.
- Eye discharge.
- Bleeding in the eye.
- Eye is bothered by bright light.
- Red eyes.
- The chance of heart attack or stroke due to blood clots may be raised. Call your doctor right away if you have signs of heart attack like chest pain that may spread to the arms, neck, jaw, back, or stomach; abnormal sweating; or feeling sick or throwing up. Call your doctor right away if you have signs of stroke like weakness on 1 side of the body; eyesight, speech, or balance problems; drooping on 1 side of the face; feeling confused; or severe headache.
What are some other side effects of ranibizumab?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Dry eyes.
- Seeing floaters.
- Feeling that something is in the eye.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out ranibizumab?
- If you need to store ranibizumab at home, talk with your doctor, nurse, or pharmacist about how to store it.
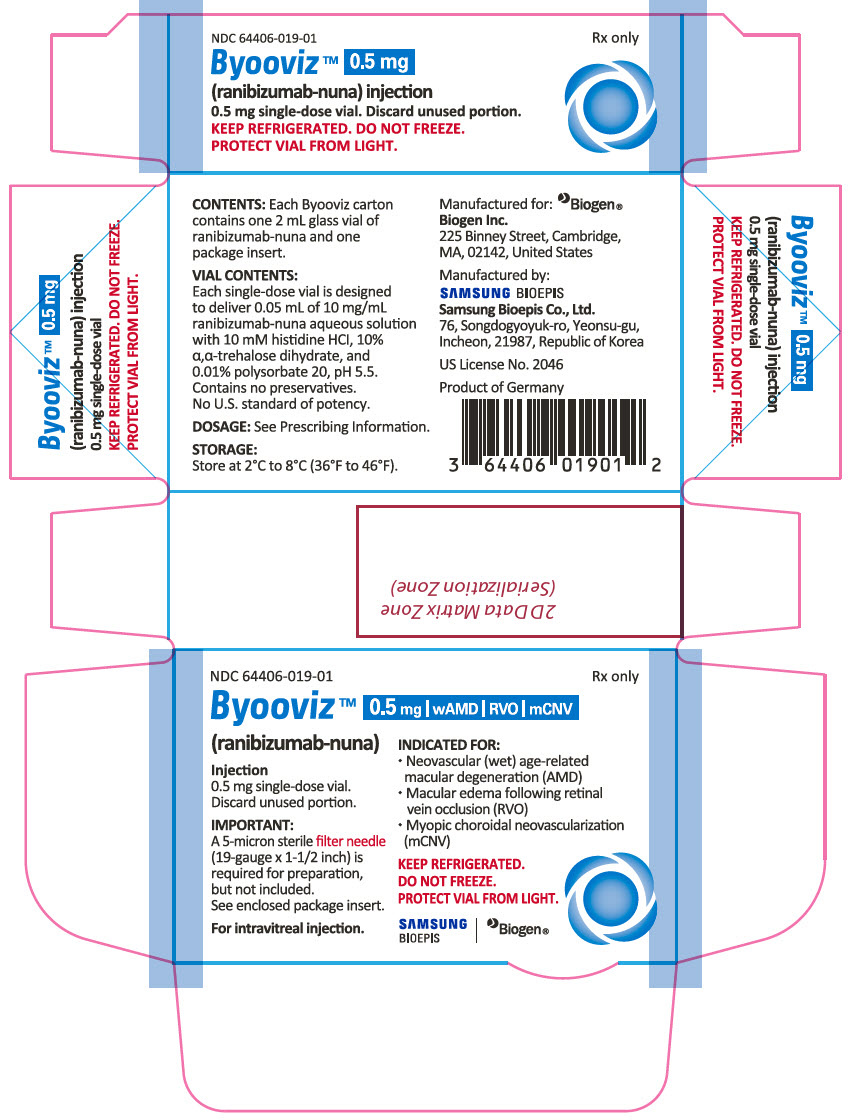
Label
PRINCIPAL DISPLAY PANEL – 0.5 MG VIAL CARTON
- NDC 64406-019-01
- Rx only
- Byooviz™ 0.5mg wAMD RVO mCNO
- (ranibizumab-nuna)
- Injection
- 0.5 mg single-dose vial.
- Discard unused portion.
- IMPORTANT:
- A 5-micron sterile filter needle (19-gauge x 1-1/2 inch) is required for preparation, but not included.
- See enclosed package insert.
- For intravitreal injection.
- INDICATED FOR:
- Neovascular (wet) age-related macular degeneration (AMD)
- Macular edema following retinal vein occlusion (RVO)
- Myopic choroidal neovascularization (mCNV)
- KEEP REFRIGERATED.
- DO NOT FREEZE.
- PROTECT VIAL FROM LIGHT.
- SAMSUNG BIOEPIS, Biogen®
SRC: NLM .
