Silenor
Generic name: doxepin
Drug classes: Miscellaneous anxiolytics, sedatives and hypnotics, Tricyclic antidepressants
What is Silenor?
Silenor is a prescription medicine that is used to treat people who have trouble staying asleep.
Description
SILENOR (doxepin) is available in 3 mg and 6 mg strength tablets for oral administration. Each tablet contains 3.39 mg or 6.78 mg doxepin hydrochloride, equivalent to 3 mg and 6mg of doxepin, respectively.
Chemically, doxepin hydrochloride is an (E) and (Z) geometric, isomeric mixture of 1 propanamine, 3-dibenz[b,e]oxepin-11(6H)ylidene-N,N-dimethyl-hydrochloride. It has the following structure:
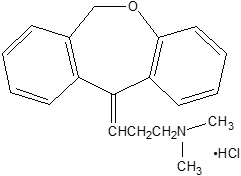
Doxepin hydrochloride is a white crystalline powder, with a slight amine-like odor, that is readily soluble in water. It has a molecular weight of 315.84 and molecular formula of C19 H21 NO∙HCl.
Each SILENOR tablet includes the following inactive ingredients: microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate. The 3 mg tablet also contains FD&C Blue No.1. The 6 mg tablet also contains D&C Yellow No. 10 and FD&C Blue No. 1.
Mechanism of Action
The mechanism of action of doxepin in sleep maintenance is unclear; however, doxepin’s effect could be mediated through antagonism of the H1 receptor.
What is the most important information I should know about Silenor?
After taking Silenor, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with Silenor. Reported activities include:
- driving a car (“sleep-driving”)
- making and eating food
- talking on the phone
- having sex
- sleep-walking
Call your healthcare provider right away if you find out that you have done any of the above activities after taking Silenor.
Important:
1. Take Silenor exactly as prescribed
- Do not take more Silenor than prescribed.
- Take Silenor 30 minutes before bedtime. After taking Silenor, you should only do activities needed to get ready for bed.
2. Do not take Silenor:
- with alcohol
- if you take other medicines that can make you sleepy. Talk to your healthcare provider about all of your medicines. Your healthcare provider will tell you if you can take Silenor with your other medicines
- if you cannot get a full night of sleep before you must be active again
Who should not take Silenor?
Do not take Silenor if you:
- take a monoamine oxidase inhibitor (MAOI) medicine or have taken an MAOI in the last 14 days (2 weeks). Ask your healthcare provider if you are not sure if your medicine is an MAOI.
- have an eye problem called narrow angle glaucoma that is not being treated
- have trouble urinating
- are allergic to any of the ingredients in Silenor. See the end of this Medication Guide for a complete list of ingredients in Silenor.
Talk to your healthcare provider before taking this medicine if you have any of these conditions.
It is not known if Silenor is safe and effective in children.
What should I tell my healthcare provider before taking Silenor?
Before you take Silenor, tell your healthcare provider if you:
- See “Who should not take Silenor?”
- have a history of depression, mental illness, or suicidal thoughts
- have severe sleep apnea
- have kidney or liver problems
- have a history of drug or alcohol abuse or addiction
- have a history of glaucoma or urinary retention
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Silenor will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. Silenor can pass into your milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take Silenor. You should not breast-feed while taking Silenor.
Tell your doctor about all of the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements.
Silenor and other medicines may affect each other causing side effects. Silenor may affect the way other medicines work, and other medicines may affects how Silenor works. Especially tell your healthcare provider if you take:
- a monoamine oxidase inhibitor (MAOI). See “Who should not take Silenor?”.
- cimetidine (Tagamet) or other medicines that can affect certain liver enzymes
- certain allergy medicines (antihistamines) or other medicines that can make you sleepy or affect your breathing
- the diabetes medicine tolazamide
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How should I take Silenor?
- Take Silenor exactly as your healthcare provider tells you to take it.
- Your doctor will tell you how many Silenor to take and when to take them.
- Your doctor may change your dose if needed.
- Take Silenor within 30 minutes of bedtime. After taking Silenor, you should confine your activities to those necessary to prepare for bed.
- Do not take Silenor within 3 hours of a meal. Silenor may not work as well, or may make you sleepy the next day if taken with or right after a meal.
- Do not take Silenor unless you are able to get a full night of sleep before you must be active again.
- Call your doctor if your sleep problems get worse or do not get better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- If you take too much Silenor, call your doctor or get medical help right away.
What should I avoid while taking Silenor?
- You should not drink alcohol while taking Silenor. Alcohol can increase your chances of getting serious side effects with Silenor.
- You should not drive, operate heavy machinery, or do other dangerous activities after Silenor.
You may still feel drowsy the next day after taking Silenor. Do not drive or do other dangerous activities after taking Silenor until you feel fully awake.
What are the possible side effects of Silenor?
Silenor can cause serious side effects including:
- See “What is the most important information I should know about Silenor?”
The most common side effect of Silenor is drowsiness or tiredness, nausea.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Silenor. For more information ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of Silenor
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Silenor for a condition for which it was not prescribed. Do not share Silenor with other people, even if you think they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Silenor. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Silenor that is written for healthcare professionals.
For more information, contact Currax Pharmaceuticals LLC at 1-800-793-2145.
How should I store Silenor?
- Store Silenor between 68° and 77° F (20° to 25°C).
- Keep Silenor in a tightly closed container, and away from light. Safely throw away medicine that is out of date or no longer needed.
- Keep Silenor and all medicines out of the reach of children.
What are the ingredients in Silenor?
Active Ingredient: doxepin hydrochloride
Inactive Ingredients: Microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate. The 3 mg tablet also contains FD&C Blue No. 1. The 6 mg tablet also contains FD&C Yellow No. 10 and FD&C Blue No. 1.
Label
PRINCIPAL DISPLAY PANEL – 3 mg Tablet Bottle Label
- 3mg
- 30 tablets
Rx Only - silenor®
doxepin tablets - NDC 42847-103-30

PRINCIPAL DISPLAY PANEL – 6 mg Tablet Bottle Label
- 6mg
- 30 tablets
Rx Only - silenor®
doxepin tablets - NDC 42847-106-30

SRC: NLM .
