Scemblix
Generic name: asciminib
Dosage form: tablets
Drug class: BCR-ABL tyrosine kinase inhibitors
Medically reviewed by A Ras MD.
What is Scemblix?
Scemblix is a prescription medicine used to treat adults with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP), previously treated with 2 or more tyrosine kinase inhibitor (TKI) medicines, Ph+ CML in CP with the T315I mutation.
It is not known if Scemblix is safe and effective in children.
Description
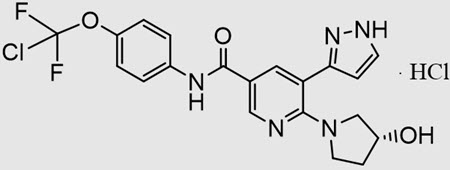
SCEMBLIX (asciminib) is a kinase inhibitor. The chemical name of the drug substance is N-[4-(Chlorodifluoromethoxy)phenyl]-6-[(3R)-3-hydroxypyrrolidin-1-yl]-5-(1H-pyrazol-3-yl)pyridine-3-carboxamide-hydrogen chloride (1/1). Asciminib hydrochloride is a white to slightly yellow powder. The molecular formula of asciminib hydrochloride is C20H18ClF2N5O3.HCl, and the relative molecular mass is 486.30 g/mol for the hydrochloride salt and 449.84 g/mol for the free base. The chemical structure of asciminib hydrochloride is shown below:
SCEMBLIX film-coated tablets are supplied for oral use with two strengths that contain 20 mg and 40 mg of asciminib (equivalent to 21.62 mg and 43.24 mg, respectively, of asciminib hydrochloride). The tablets contain colloidal silicon dioxide, croscarmellose sodium, ferric oxide, hydroxypropyl cellulose, lactose monohydrate, lecithin, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, talc, titanium dioxide, and xanthan gum. The 20 mg tablets contain ferric oxide, yellow and ferric oxide, red. The 40 mg tablets contain ferrosoferric oxide and ferric oxide, red.
Mechanism of Action
Asciminib is an ABL/BCR-ABL1 tyrosine kinase inhibitor. Asciminib inhibits the ABL1 kinase activity of the BCR-ABL1 fusion protein, by binding to the ABL myristoyl pocket. In studies conducted in vitro or in animal models of CML, asciminib showed activity against wild-type BCR-ABL1 and several mutant forms of the kinase, including the T315I mutation.
What should I tell my healthcare provider before taking Scemblix?
Before taking Scemblix, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of inflammation of your pancreas (pancreatitis)
- have a history of heart problems or blood clots in your arteries and veins (types of blood vessels)
- are pregnant or plan to become pregnant. Scemblix can harm your unborn baby.
- Your healthcare provider will do a pregnancy test before you start treatment with Scemblix.
- Females who are able to become pregnant should use effective birth control during treatment and for 1 week after your last dose of Scemblix. Talk to your healthcare provider about birth control methods that may be right for you.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Scemblix.
- are breastfeeding or plan to breastfeed. It is not known if Scemblix passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose of Scemblix.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Scemblix and other medicines may affect each other causing side effects.
How should I take Scemblix?
- Take Scemblix exactly as your healthcare provider tells you.
- Do not change your dose or schedule or stop taking Scemblix unless your healthcare provider tells you to.
- Take Scemblix without food. You should avoid eating for at least 2 hours before and 1 hour after taking Scemblix.
- Swallow Scemblix tablets whole. Do not break, crush, or chew Scemblix tablets.
- If you take Scemblix 1 time a day and miss a dose by more than 12 hours, skip the missed dose and take your next dose at your regular time.
- If you take Scemblix 2 times a day and miss a dose by more than 6 hours, skip the missed dose and take your next dose at your regular time.
What are the possible side effects of Scemblix?
Scemblix may cause serious side effects, including:
- Low blood cell counts. Scemblix may cause low platelet counts (thrombocytopenia), low white blood cell counts (neutropenia), and low red blood cell counts (anemia). Your healthcare provider will do blood tests to check your blood cell counts every 2 weeks for the first 3 months of treatment and then monthly or as needed during treatment with Scemblix. Tell your healthcare provider right away if you have unexpected bleeding or easy bruising, blood in your urine or stools, fever, or any signs of an infection.
- Pancreas problems. Scemblix may increase enzymes in your blood called amylase and lipase, which may be a sign of pancreatitis. Your healthcare provider may do blood tests monthly or as needed during treatment with Scemblix to check for problems with your pancreas. Tell your healthcare provider right away if you have sudden stomach-area pain or discomfort, nausea, or vomiting.
- High blood pressure. Your healthcare provider may check your blood pressure and treat any high blood pressure during treatment with Scemblix as needed. Tell your healthcare provider if you develop elevated blood pressure or symptoms of high blood pressure including confusion, headaches, dizziness, chest pain, or shortness of breath.
- Allergic reaction. Stop taking Scemblix and get medical help right away if you get any signs or symptoms of an allergic reaction, including:
- trouble breathing or swallowing
- swelling of the face, lips, or tongue
- skin rash or flushing of your skin
- feeling dizzy or faint
- fever
- fast heartbeat
- Heart and blood vessel (cardiovascular) problems. Scemblix may cause heart and blood vessel problems, including heart attack, stroke, blood clots or blockage of your arteries, heart failure, and abnormal heartbeat, which can be serious and may sometimes lead to death. These heart and blood vessel problems can happen in people with risk factors or a history of these problems, and/or previously treated with other TKI medicines. Your healthcare provider may monitor you for heart and blood vessel problems and treat you as needed during treatment with Scemblix. Tell your healthcare provider or get medical help right away if you get:
- shortness of breath
- chest pain or pressure
- feeling like your heart is beating too fast or you feel abnormal heartbeats
- swelling in your ankles or feet
- dizziness
- weight gain
- numbness or weakness on one side of your body
- decreased vision or loss of vision
- trouble talking
- pain in your arms, legs, back, neck or jaw
- headache
- severe stomach area pain
The most common side effects of Scemblix include:
- nose, throat, or sinus (upper respiratory tract) infections
- muscle, bone, or joint pain
- tiredness
- nausea
- rash
- diarrhea
- decreased blood platelet counts, white blood cell counts, and red blood cell counts
- increased blood fat (triglycerides) levels
- increased blood creatine kinase levels
- increased blood liver enzyme levels
- increased blood pancreas enzyme (amylase and lipase) levels
Your healthcare provider may change your dose or temporarily or permanently stop treatment with Scemblix if you have certain side effects.
Scemblix may cause fertility problems in females. This may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Scemblix.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Scemblix
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Scemblix for a condition for which it was not prescribed. Do not give Scemblix to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for more information about Scemblix that is written for health professionals.
How should I store Scemblix?
- Store Scemblix at room temperature between 68°F to 77°F (20°C to 25°C).
- Dispense and store Scemblix in the original container to protect it from moisture.
Keep Scemblix and all medicines out of the reach of children.
What are the ingredients in Scemblix?
Active ingredient: asciminib
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, ferric oxide, hydroxypropyl cellulose, lactose monohydrate, lecithin, magnesium stearate, microcrystalline cellulose, polyvinyl alcohol, talc, titanium dioxide, and xanthan gum. The 20 mg tablets contain ferric oxide, yellow and ferric oxide, red. The 40 mg tablets contain ferrosoferric oxide and ferric oxide, red.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-1098-20
- SCEMBLIX®
- (asciminib)
Tablets - 40 mg*
- Rx only
- 60 Tablets
- Swallow tablets whole. Do not break,
crush, or chew the tablets. - NOVARTIS
SRC: NLM .
