Sarclisa
Generic name: isatuximab-irfc
Dosage form: injection, for intravenous use
Drug class: CD38 monoclonal antibodies
Medically reviewed by A Ras MD.
What is Sarclisa?
Sarclisa is a prescription medicine used in combination with pomalidomide and dexamethasone to treat adults who have received at least 2 prior therapies, including lenalidomide and a proteasome inhibitor, to treat multiple myeloma.
It is not known if Sarclisa is safe and effective in children.
Description
Isatuximab-irfc, a CD38-directed cytolytic antibody, is a chimeric immunoglobulin G1 (IgG1) monoclonal antibody (mAb). Isatuximab-irfc is produced from a mammalian cell line (Chinese hamster ovary, CHO) using a fed-batch production process. Isatuximab-irfc is composed of two identical immunoglobulin kappa light chains and two identical immunoglobulin gamma heavy chains and has an overall molecular weight of approximately 148 kDa.
SARCLISA (isatuximab-irfc) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution, essentially free of visible particles in a single-dose vial for intravenous use. Each vial contains either 100 mg/5 mL or 500 mg/25 mL of isatuximab-irfc at a concentration of 20 mg/mL with a pH of 6.0. Each mL of solution contains 20 mg isatuximab-irfc, histidine (1.46 mg), histidine hydrochloride monohydrate (2.22 mg), polysorbate 80 (0.2 mg), sucrose (100 mg), and water for injection.
Mechanism of Action
Isatuximab-irfc is an IgG1-derived monoclonal antibody that binds to CD38 expressed on the surface of hematopoietic and tumor cells, including multiple myeloma cells. Isatuximab-irfc induces apoptosis of tumor cells and activation of immune effector mechanisms including antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement dependent cytotoxicity (CDC). Isatuximab-irfc inhibits the ADP-ribosyl cyclase activity of CD38. Isatuximab-irfc can activate natural killer (NK) cells in the absence of CD38-positive target tumor cells and suppresses CD38-positive T-regulatory cells. The combination of isatuximab-irfc and pomalidomide enhanced ADCC activity and direct tumor cell killing compared to that of isatuximab-irfc alone in vitro, and enhanced antitumor activity compared to the activity of isatuximab-irfc or pomalidomide alone in a human multiple myeloma xenograft model.
What is the most important information I should know about Sarclisa?
Sarclisa is used in combination with other medicines called pomalidomide and dexamethasone. You should also read the Medication Guide that comes with pomalidomide. You can ask your healthcare provider or pharmacist for information about dexamethasone.
Who should not use Sarclisa?
Do not receive Sarclisa if you have a history of a severe allergic reaction to isatuximab-irfc or any of the ingredients in Sarclisa. See the end of this guide for complete list of ingredients in Sarclisa.
What should I tell my healthcare provider before using Sarclisa?
Before receiving Sarclisa, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. Sarclisa may harm your unborn baby. You should not receive Sarclisa during pregnancy.
- Females who are able to become pregnant should use an effective method of birth control during treatment and for 5 months after your last dose of Sarclisa. Talk to your healthcare provider about birth control methods that you can use during this time.
Tell your healthcare provider right away if you think you are pregnant or become pregnant during treatment with Sarclisa.
- are breastfeeding or plan to breastfeed. It is not known if Sarclisa passes into your breast milk. You should not breastfeed during treatment with Sarclisa.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Sarclisa?
- Sarclisa will be given to you by your healthcare provider by intravenous (IV) infusion into your vein.
- Sarclisa is given in treatment cycles of 28 days (4 weeks), together with the medicines pomalidomide and dexamethasone.
- In cycle 1, Sarclisa is usually given weekly.
- Starting in cycle 2, Sarclisa is usually given every 2 weeks.
Your healthcare provider will decide how long you should receive Sarclisa.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
- Your healthcare provider will give you medicines before each dose of Sarclisa, to help reduce the risk of infusion reactions (make them less frequent and severe).
What are the possible side effects of Sarclisa?
Sarclisa may cause serious side effects including:
- Infusion reactions. Infusion reactions are common with Sarclisa and can sometimes be severe.
- Your healthcare provider will prescribe medicines before each infusion of Sarclisa to help decrease your risk for infusion reactions or to help make any infusion reaction less severe. You will be monitored for infusion reactions during each dose of Sarclisa.
- Your healthcare provider may slow down or stop your infusion, or completely stop treatment with Sarclisa if you have an infusion reaction.
Tell your healthcare provider right away if you develop any of the following symptoms of infusion reaction during or within 24 hours after an infusion of Sarclisa:
- feeling short of breath
- cough
- chills
- nausea
- Decreased white blood cell counts. Decreased white blood cell counts are common with Sarclisa and certain white blood cells can be severely decreased. You may have an increased risk of getting certain infections, such as upper and lower respiratory infections.
Your healthcare provider will check your blood cell counts during treatment with Sarclisa. Your healthcare provider may prescribe an antibiotic or antiviral medicine to help prevent infection, or a medicine to help increase your white blood cell counts during treatment with Sarclisa.
Tell your healthcare provider right away if you develop any fever or symptoms of infection during treatment with Sarclisa. - Risk of new cancers. New cancers have happened in people during treatment with Sarclisa. Your healthcare provider will monitor you for new cancers during treatment with Sarclisa.
- Change in blood tests. Sarclisa can affect the results of blood tests to match your blood type. Your healthcare provider will do blood tests to match your blood type before you start treatment with Sarclisa. Tell all of your healthcare providers that you are being treated with Sarclisa before receiving blood transfusions.
The most common side effects of Sarclisa include:
- lung infection (pneumonia)
- decreased red blood cell counts (anemia)
- upper respiratory tract infection
- decreased platelet counts (thrombocytopenia)
- diarrhea
These are not all the possible side effects of Sarclisa. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Sarclisa
Medicines are sometimes prescribed for purposes other than those listed in a Patient information guide. You can ask your pharmacist or healthcare provider for information about Sarclisa that is written for health professionals.
How should I store Sarclisa?
Store in a refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light. Do not freeze. Do not shake.
What are the ingredients in Sarclisa?
Active ingredient: isatuximab-irfc
Inactive ingredients: histidine, histidine hydrochloride monohydrate, polysorbate 80, sucrose, and water for injection.
Label
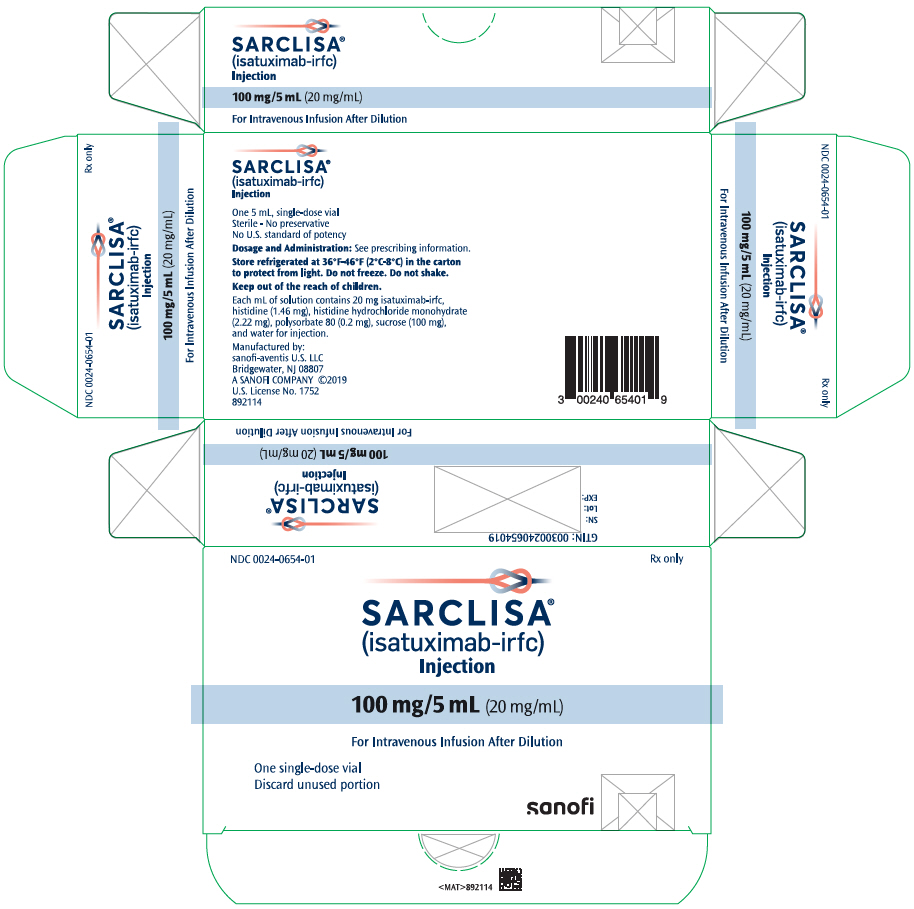
PRINCIPAL DISPLAY PANEL – 100 MG/5 ML VIAL CARTON
- NDC 0024-0654-01
Rx only - SARCLISA®
(isatuximab-irfc)
Injection - 100 mg/5 mL (20 mg/mL)
- For Intravenous Infusion After Dilution
- One single-dose vial
Discard unused portion - SANOFI
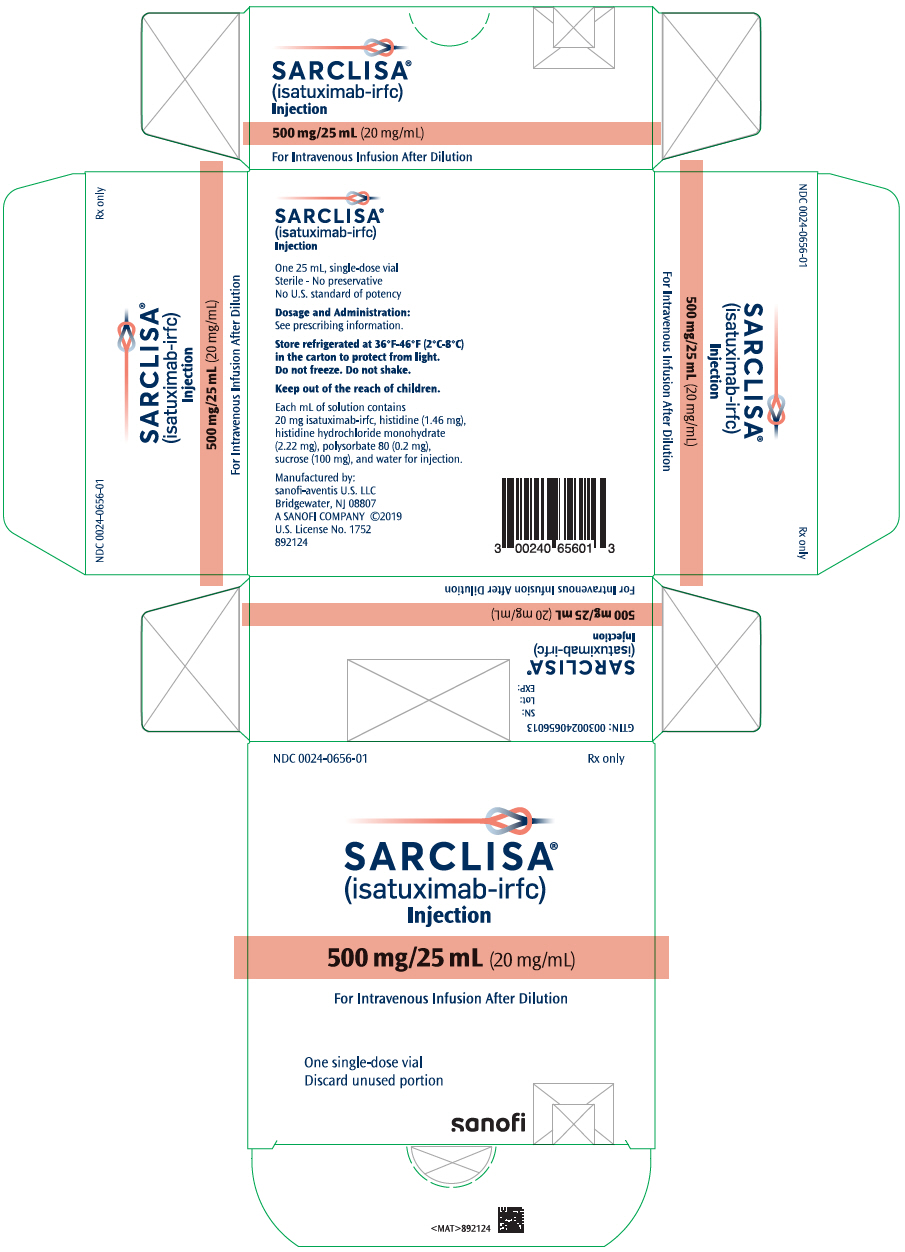
PRINCIPAL DISPLAY PANEL – 500 MG/25 ML VIAL CARTON
- NDC 0024-0656-01
Rx only - SARCLISA®
(isatuximab-irfc)
Injection - 500 mg/25 mL (20 mg/mL)
- For Intravenous Infusion After Dilution
- One single-dose vial
Discard unused portion - SANOFI
SRC: NLM .
