Sabril
Generic name: vigabatrin
Brand names: Sabril, Vigadrone
Drug class: Gamma-aminobutyric acid analogs
Medically reviewed by A Ras MD
What is Sabril?
Sabril is a prescription medicine used along with other treatments to treat adults and children 2 years and older with complex partial seizures (CPS) if the CPS do not respond well enough to several other treatments, and you and your healthcare provider decide the possible benefit of taking Sabril is more important than the risk of vision loss.
Sabril should not be the first medicine used to treat CPS
Sabril is also used to treat babies 1 month to 2 years of age who have infantile spasms (IS) if you and your healthcare provider decide the possible benefits of taking Sabril are more important than the possible risk of vision loss.
Description
SABRIL (vigabatrin) is an oral antiepileptic drug and is available as white film-coated 500 mg tablets and as a white to off-white granular powder for oral solution in packets of 500 mg.
The chemical name of vigabatrin, a racemate consisting of two enantiomers, is (±) 4-amino-5-hexenoic acid. The molecular formula is C6H11NO2 and the molecular weight is 129.16. It has the following structural formula:
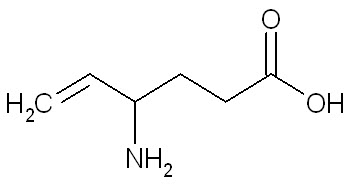
Vigabatrin is a white to off-white powder which is freely soluble in water, slightly soluble in methyl alcohol, very slightly soluble in ethyl alcohol and chloroform, and insoluble in toluene and hexane. The pH of a 1% aqueous solution is about 6.9. The n-octanol/water partition coefficient of vigabatrin is about 0.011 (log P=-1.96) at physiologic pH. Vigabatrin melts with decomposition in a 3-degree range within the temperature interval of 171ºC to 176ºC. The dissociation constants (pKa) of vigabatrin are 4 and 9.7 at room temperature (25ºC).
Each SABRIL tablet contains 500 mg of vigabatrin. The inactive ingredients are hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycols, povidone, sodium starch glycolate, and titanium dioxide.
SABRIL for oral solution is available as a white to off-white granular powder. Each packet contains 500 mg of vigabatrin. The inactive ingredient is povidone.
Mechanism of Action
The precise mechanism of vigabatrin’s anti-seizure effect is unknown, but it is believed to be the result of its action as an irreversible inhibitor of γ-aminobutyric acid transaminase (GABA-T), the enzyme responsible for the metabolism of the inhibitory neurotransmitter GABA. This action results in increased levels of GABA in the central nervous system.
No direct correlation between plasma concentration and efficacy has been established. The duration of drug effect is presumed to be dependent on the rate of enzyme re-synthesis rather than on the rate of elimination of the drug from the systemic circulation.
What is the most important information I should know about Sabril?
Sabril can cause serious side effects, including:
- Permanent vision loss
- Magnetic resonance imaging (MRI) changes in babies with infantile spasms (IS)
- Risk of suicidal thoughts or actions
1. Permanent vision loss:
Sabril can damage the vision of anyone who takes it. Some people can have severe loss particularly to their ability to see to the side when they look straight ahead (peripheral vision). With severe vision loss, you may only be able to see things straight in front of you (sometimes called “tunnel vision”). You may also have blurry vision. If this happens, it will not get better.
- Vision loss and use of Sabril in adults and children 2 years and older: Because of the risk of vision loss, Sabril is used to treat complex partial seizures (CPS) only in people who do not respond well enough to several other medicines.Tell your healthcare provider right away if you (or your child):
- might not be seeing as well as before starting Sabril.
- start to trip, bump into things, or are more clumsy than usual.
- are surprised by people or things coming in front of you that seem to come out of nowhere.
- These changes can mean that you (or your child) have damage to your vision.
- It is recommended that your healthcare provider test your (or your child’s) vision (including peripheral vision) and visual acuity (ability to read an eye chart) before you (or your child) start Sabril or within 4 weeks after starting Sabril, and at least every 3 months after that until Sabril is stopped. It is also recommended that you (or your child) have a vision test about 3 to 6 months after Sabril is stopped. Your vision loss may get worse after you stop taking Sabril.
- Some people are not able to complete testing of vision. Your healthcare provider will determine if you (or your child) can be tested. If you (or your child) cannot complete vision testing, your healthcare provider may continue prescribing Sabril, but your healthcare provider will not be able to watch for any vision loss you (or your child) may get.
- Even if your vision (or your child’s vision) seems fine, it is important that you (or your child) get these regular vision tests because vision damage can happen before you (or your child) notice any changes.
- These vision tests cannot prevent the vision damage that can happen with Sabril, but they do allow the healthcare provider to decide if you (or your child) should stop Sabril if your vision has gotten worse.
- Vision testing may not detect vision loss before it is severe.
- If you do not have these vision tests regularly, your healthcare provider may stop prescribing Sabril.
- If you drive and your vision is damaged by Sabril, driving might be more dangerous, or you may not be able to drive safely at all. Talk about this with your healthcare provider.
- Vision loss in babies: Because of the risk of vision loss, Sabril is used in babies 1 month to 2 years of age with infantile spasms (IS) only when you and your healthcare provider decide that the possible benefits of Sabril are more important than the risks.
- Parents or caregivers are not likely to recognize the symptoms of vision loss in babies until it is severe. Healthcare providers may not find vision loss in babies until it is severe.
- It is difficult to test vision in babies, but, to the extent possible, all babies should have their vision tested before starting Sabril or within 4 weeks after starting Sabril, and every 3 months after that until Sabril is stopped. Your baby should also have a vision test about 3 to 6 months after Sabril is stopped.
- Your baby may not be able to be tested. Your healthcare provider will determine if your baby can be tested. If your baby cannot be tested, your healthcare provider may continue prescribing Sabril, but your healthcare provider will not be able to watch for any vision loss.
Tell your healthcare provider right away if you think that your baby is:
- not seeing as well as before taking Sabril.
- acting differently than normal.
- Even if your baby’s vision seems fine, it is important to get regular vision tests because damage can happen before your baby acts differently. Even these regular vision exams may not show the damage to your baby’s vision before it is severe and permanent.
All people who take Sabril:
|
- Because Sabril might cause permanent vision loss, it is available to healthcare providers and patients only under a special program called the Vigabatrin Risk Evaluation and Mitigation Strategy (REMS) Program. Sabril can only be prescribed to people who are enrolled in this program. As part of the Vigabatrin REMS Program, it is recommended that your healthcare provider test your (or your child’s) vision from time to time (periodically) while you (or your child) are being treated with Sabril, and even after you (or your child) stop treatment. Your healthcare provider will explain the details of the Vigabatrin REMS Program to you. For more information, go to www.vigabatrinREMS.com or call 1-866-244-8175.
2. Magnetic resonance imaging (MRI) changes in babies with infantile spasms:
Brain pictures taken by magnetic resonance imaging (MRI) show changes in some babies after they are given Sabril. It is not known if these changes are harmful.
3. Risk of suicidal thoughts or actions:
Like other antiepileptic drugs, Sabril may cause suicidal thoughts or actions in a very small number of people, about 1 in 500 people taking it. Call a healthcare provider right away if you or your child have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
Suicidal thoughts or actions can be caused by things other than medicines. If you or your child have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
- Do not stop Sabril without first talking to a healthcare provider.
- Stopping Sabril suddenly can cause serious problems. Stopping a seizure medicine suddenly can cause seizures that will not stop (status epilepticus) in people who are being treated for seizures.
What should I tell my healthcare provider before taking Sabril?
If you or your child has CPS, before taking Sabril tell your healthcare provider about all of your medical conditions, including if you or your child:
- have or had an allergic reaction to Sabril, such as hives, itching, or trouble breathing.
- have or had any vision problems.
- have or had any kidney problems.
- have or had low red blood cell counts (anemia).
- have or had any nervous or mental illnesses such as depression, mood problems, thoughts of suicide, or attempts at suicide.
- are breastfeeding or planning to breastfeed. Sabril can pass into breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take Sabril.
- are pregnant or plan to become pregnant. Sabril can cause harm to your unborn baby. You and your healthcare provider will have to decide if you should take Sabril while you are pregnant.
Pregnancy Registry:
If you become pregnant while taking Sabril, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/. The purpose of this registry is to collect information about the safety of antiepileptic medicine during pregnancy.
If you are a parent or caregiver whose baby has IS, before giving Sabril to your baby, tell your healthcare provider about all of your baby’s medical conditions, including if your baby has or ever had:
- an allergic reaction to Sabril, such as hives, itching, or trouble breathing.
- any vision problems.
- any kidney problems.
Tell your healthcare provider about all the medicines you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Sabril and other medicines may affect each other causing side effects.
How should I take Sabril?
- Sabril comes as tablets or powder for oral solution.
- You or your child will receive Sabril from a specialty pharmacy.
- Take Sabril exactly as your healthcare provider tells you to. Sabril is usually taken 2 times each day.
- Sabril may be taken with or without food.
- Before starting to take Sabril, talk to your healthcare provider about what you or your child should do if a Sabril dose is missed.
- If you or your child are taking Sabril for CPS and the seizures do not improve enough within 3 months, your healthcare provider will stop prescribing Sabril.
- If your child is taking Sabril for IS and the seizures do not improve within 2 to 4 weeks, your healthcare provider will stop prescribing Sabril.
- Do not stop taking Sabril suddenly. This can cause serious problems. Stopping Sabril or any seizure medicine suddenly can cause seizures that will not stop (status epilepticus) in people who are being treated for seizures. You should follow your healthcare provider’s instructions on how to stop taking Sabril.
- Tell your healthcare provider right away about any increase in seizures when Sabril treatment is being stopped. Before your child starts taking Sabril, speak to your child’s healthcare provider about what to do if your baby misses a dose, vomits, spits up, or only takes part of the dose of Sabril.
- Do not stop taking Sabril without talking to your healthcare provider. If Sabril improves your (or your child’s) seizures, you and your healthcare provider should talk about whether the benefit of taking Sabril is more important than the risk of vision loss, and decide if you (or your child) will continue to take Sabril.
- If you are giving Sabril powder for oral solution to your child, it can be given at the same time as their meal. Sabril for oral solution powder should be mixed with water only.
- See Instructions for Use for detailed information about how to mix and give Sabril powder for oral solution to your child the right way.
What should I avoid while taking Sabril?
Sabril causes sleepiness and tiredness. Adults taking Sabril should not drive, operate machinery, or perform any hazardous task, unless you and your healthcare provider have decided that you can do these things safely.
What are the possible side effects of Sabril?
Sabril can cause serious side effects, including:
- See “What is the most important information I should know about Sabril?”
- sleepiness and tiredness. See “What should I avoid while taking Sabril?”
- Sabril may cause your baby to be sleepy. Sleepy babies may have a harder time suckling and feeding, or may be irritable.
- weight gain that happens without swelling.
The following serious side effects happen in adults. It is not known if these side effects also happen in babies who take Sabril.
- low red blood cell counts (anemia).
- nerve problems. Symptoms of a nerve problem can include numbness and tingling in your toes or feet. It is not known if nerve problems will go away after you stop taking Sabril.
- swelling.
If you or your child has CPS, Sabril may make certain types of seizures worse. Tell your healthcare provider right away if your (or your child’s) seizures get worse.
The most common side effect of Sabril in adults include blurred vision, sleepiness, dizziness, problems walking or feeling uncoordinated, shaking (tremor), and tiredness.
The most common side effect of Sabril in children 3 to 16 years of age is weight gain. Also expect side effects like those seen in adults.
If you are giving Sabril to your baby for IS:
Sabril may make certain types of seizures worse. You should tell your baby’s healthcare provider right away if your baby’s seizures get worse. Tell your baby’s healthcare provider if you see any changes in your baby’s behavior.
The most common side effects of Sabril in babies include:
- sleepiness – Sabril may cause your baby to be sleepy. Sleepy babies may have a harder time suckling and feeding or may be irritable.
- swelling in the bronchial tubes (bronchitis)
- ear infection
- irritability
- nausea
Tell your healthcare provider if you or your child have any side effect that bothers you or that does not go away. These are not all the possible side effects of Sabril.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Sabril
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Sabril that is written for health professionals. Do not use Sabril for a condition for which it was not prescribed. Do not give Sabril to other people, even if they have the same symptoms that you have. It may harm them.
How should I store Sabril?
- Store Sabril tablets and Sabril packets at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
Keep Sabril and all medicines out of the reach of children.
What are the ingredients in Sabril?
Active Ingredient: vigabatrin
Inactive Ingredients:
Tablets: hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycols, povidone, sodium starch glycolate, and titanium dioxide
Powder for oral solution: povidone
For more information, go to www.SABRIL.net or call 1-866-402-8520.
Instructions for use for Sabril
Powder for oral solution
Important Note:
- Sabril comes in a packet
- Each packet contains 500 mg of Sabril powder
- Sabril powder must be mixed with water only.The water may be cold or at room temperature.
- Your healthcare provider will tell you:
- how many packets of Sabril you will need for each dose
- how many milliliters (mL) of water to use to mix one dose of Sabril
- how many milliliters (mL) of the powder and water mixture you will need for each dose of medicine
- Sabril should be given right away after it is mixed
- Use the oral syringes, provided by the pharmacy, to measure and give the correct dose. Do not use a household teaspoon or tablespoon.
Supplies you will need to mix 1 dose of Sabril:
- The number of packets of Sabril needed for each dose
- 2 clean cups: 1 for mixing and 1 for water. The cup used for mixing Sabril should be clear so you can see if the powder is dissolved
- Water to mix with the Sabril powder
- One small 3 mL oral syringe and one large 10 mL oral syringe which are provided by the pharmacy
- Small spoon or other clean utensil to stir the mixture
- Scissors
Step 1: Start with 1 of the empty cups and the total number of packets you will need for 1 dose.
Step 2: Before you open the packet, tap it to settle all the powder to the bottom of the packet.
Step 3: Use a pair of scissors to cut open the Sabril packet along the dotted line.
Step 4: Empty the entire contents of the Sabril packet into 1 of the clean empty cups (see Figure A).

- Repeat steps 2 to 4 above to open all of the packets needed for 1 dose of Sabril.
Step 5: Take the second cup and fill it half way with water (see Figure B).
Do not mix Sabril with anything other than water.

- You will use the larger oral syringe (10 mL) to draw up the water needed to mix with the powder from the packets. You will need 10 mL of water for each packet of Sabril.For example:
- If you are using 1 packet of Sabril, you will need to use 10 mL of water (fill the 10 mL oral syringe 1 time)
- If you are using 2 packets of Sabril, you will need to use 20 mL of water (fill the 10 mL oral syringe 2 times)
- If you are using 3 packets of Sabril, you will need to use 30 mL of water (fill the 10 mL oral syringe 3 times)
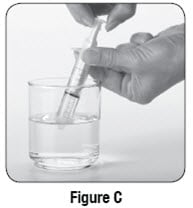
Step 6: Use the 10 mL oral syringe to draw up 10 mL of water. To do this, put the tip of the oral syringe all the way into the water in your cup. Then pull the plunger up towards you until the edge of the plunger is at the 10 mL line on the barrel of the oral syringe (see Figure C).
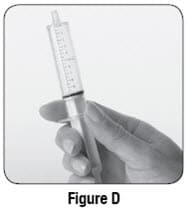
- If you see bubbles of air in the oral syringe after drawing up the water, turn the oral syringe so the tip is pointing up (see Figure D). The air will move to the top of the oral syringe. Pull the plunger back towards you and then push it back gently into the oral syringe to get rid of the bubbles.Tiny bubbles are normal.
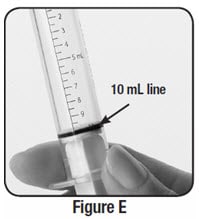
Step 7: Check the oral syringe to make sure it is filled with water up to the 10 mL line (see Figure E).
Step 8: Get the second cup that contains the Sabril needed for your dose.
Step 9: Hold the 10mL oral syringe that is filled with water with the tip pointing down over the Sabril.
Step 10: Slowly push the oral syringe plunger all the way down to empty the water from the oral syringe straight into the cup containing the Sabril (see Figure F).

Repeat steps 6 through 10 until all of the water that is needed to mix 1 dose of Sabril has been added to the cup containing the powder.
Step 11: Stir the mixture with the small spoon or other clean utensil until the solution is clear (see Figure G). This means that all of the powder is dissolved and ready for use.

- To give a dose of Sabril to your child, you should use the oral syringe to draw up the total number of mLs of the mixture that your healthcare provider tells you to.
- If you are giving 3 mL or less of the mixture, use the smaller 3 mL oral syringe.
- If you are giving more than 3 mL of the mixture, use the larger 10 mL oral syringe (this is the oral syringe that you just used to add the water).
Step 12: Put the tip of the oral syringe all the way into the mixture. Pull the plunger up towards you to draw up the mixture. Stop when the edge of the plunger lines up with markings on the barrel of the oral syringe that matches the number of mLs of mixture your healthcare provider told you to give (see Figure H).

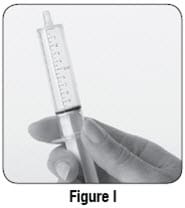
- If you see bubbles of air in the oral syringe after drawing up the mixture, turn the oral syringe so the tip is pointing up (see Figure I). The air will move to the top of the oral syringe. Pull the plunger back towards you and then gently push it back in the oral syringe in order to get rid of the bubbles. Tiny bubbles are normal.
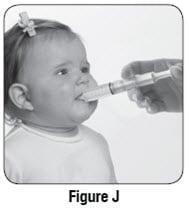
Step 13: Place the tip of the oral syringe into your child’s mouth and point the oral syringe towards either cheek (see Figure J). Push on the plunger slowly, a small amount at a time, until all of the mixture in the oral syringe is given.
- If the dose you are giving your child is more than 10 mLs, repeat steps 12 and 13 until you give the total dose of mixture prescribed by your healthcare provider.
Step 14: Throw away any mixture that is left over. Do not save or reuse any leftover mixture.
Step 15: Wash the oral syringes and mixing cups in warm water. To clean the oral syringes, remove the plunger by gently pulling it straight out of the barrel. The barrel and plunger can be hand washed with soap and water, rinsed, and allowed to dry.
SRC: NLM .
