Relistor
Generic name: methylnaltrexone (oral/injection)
Drug class: Peripheral opioid receptor antagonists
Medically reviewed by A Ras MD.
What is Relistor?
Relistor is a prescription medicine used to treat constipation in adults that is caused by prescription pain medicines called opioids.
Relistor tablets and Relistor injection are used to treat constipation caused by opioids in adults with long-lasting (chronic) pain that is not caused by active cancer.
Relistor injection is used to treat constipation caused by opioids in adults with advanced illness or pain caused by active cancer and who need increases in their opioid dose for comfort care.
It is not known if Relistor is safe and effective in children.
Description
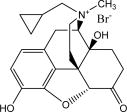
RELISTOR® (methylnaltrexone bromide) is a mu-opioid receptor antagonist. The chemical name for methylnaltrexone bromide is (R)-N-(cyclopropylmethyl) noroxymorphone methobromide. The molecular formula is C21H26NO4Br, and the molecular weight is 436.36.
The structural formula is:
RELISTOR tablets for oral administration are film-coated and contain 150 mg of methylnaltrexone bromide (equivalent to 122.5 mg methylnaltrexone). Inactive ingredients are silicified microcrystalline cellulose, microcrystalline cellulose, sodium lauryl sulfate, croscarmellose sodium, crospovidone, poloxamer 407, stearic acid (vegetable source), colloidal silicon dioxide, edetate calcium disodium, polyvinyl alcohol, titanium dioxide, polyethylene glycol and talc.
RELISTOR for subcutaneous administration is a sterile, clear and colorless to pale yellow aqueous solution. Each 3 mL vial contains 12 mg of methylnaltrexone bromide (equivalent to 9.8 mg of methylnaltrexone) in 0.6 mL of water. The excipients are 3.9 mg sodium chloride USP, 0.24 mg edetate calcium disodium USP, and 0.18 mg glycine hydrochloride. During manufacture, the pH may have been adjusted with hydrochloric acid and/or sodium hydroxide.
Each 8 mg/0.4 mL pre-filled syringe (1 mL syringe) contains 8 mg of methylnaltrexone bromide (equivalent to 6.5 mg of methylnaltrexone) in 0.4 mL of water. The excipients are 2.6 mg sodium chloride USP, 0.16 mg edetate calcium disodium USP, and 0.12 mg glycine hydrochloride.
Each 12 mg/0.6 mL pre-filled syringe (1 mL syringe) contains 12 mg of methylnaltrexone bromide (equivalent to 9.8 mg of methylnaltrexone) in 0.6 mL of water. The excipients are 3.9 mg sodium chloride USP, 0.24 mg edetate calcium disodium USP, and 0.18 mg glycine hydrochloride.
Mechanism of Action
Methylnaltrexone is a selective antagonist of opioid binding at the mu-opioid receptor. As a quaternary amine, the ability of methylnaltrexone to cross the blood-brain barrier is restricted. This allows methylnaltrexone to function as a peripherally-acting mu-opioid receptor antagonist in tissues such as the gastrointestinal tract, thereby decreasing the constipating effects of opioids without impacting opioid-mediated analgesic effects on the central nervous system (CNS).
What is the most important information I should know about Relistor?
Relistor can cause serious side effects, including:
- Tear in your stomach or intestinal wall (perforation). Stomach pain that is severe can be a sign of a serious medical condition. If you get stomach pain that is severe, does not go away, or gets worse, stop taking Relistor and get emergency medical help right away.
- Diarrhea that is severe or that will not go away. Stop taking Relistor and call your healthcare provider if you get diarrhea that is severe or that does not go away during treatment with Relistor.
- Opioid withdrawal. You may have symptoms of opioid withdrawal during treatment with Relistor including sweating, chills, diarrhea, stomach pain, anxiety, and yawning. Tell your healthcare provider if you have any of these symptoms.
Who should not take Relistor?
Do not use Relistor if you have a bowel blockage (intestinal obstruction) or have a history of bowel blockage.
What should I tell my healthcare provider before taking Relistor?
Before you start taking Relistor, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems.
- have liver problems.
- have any stomach or bowel (intestines) problems, including stomach ulcer, Crohn’s disease, diverticulitis, cancer of the stomach or bowel, or Ogilvie’s syndrome.
- are pregnant or plan to become pregnant. Taking Relistor during pregnancy may cause opioid withdrawal symptoms in your unborn baby. Tell your healthcare provider right away if you become pregnant during treatment with Relistor.
- are breastfeeding or plan to breastfeed. It is not known if Relistor passes into your breast milk. Taking Relistor while you are breastfeeding may cause opioid withdrawal in your baby. You should not breastfeed during treatment with Relistor.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Relistor?
- Stay close to a toilet after taking Relistor.
- Stop taking Relistor if you stop taking your prescription opioid pain medicine. Tell your healthcare provider if your pain medicine changes.
- If you take too much Relistor, call your healthcare provider or go to the nearest emergency room right away.
- If you take Relistor for long-lasting (chronic) pain that is not caused by cancer:
- Relistor has been shown to be effective in people who have taken opioid pain medicines for at least 4 weeks to treat long-lasting (chronic) pain not caused by cancer.
- Stop taking other laxatives before you start treatment with Relistor. You may use other laxatives if Relistor does not work after 3 days of treatment.
Tablets:
- Take Relistor tablets 1 time each day with water. Take Relistor tablets on an empty stomach at least 30 minutes before your first meal of the day.
Injection (Vials and Pre-filled Syringes):
See the detailed Instructions for Use that comes with Relistor injection for information about how to prepare and inject Relistor injection, and properly throw away (dispose of) used needles and syringes the right way.
- Relistor injection is injected under the skin (subcutaneous injection) of the upper arm, stomach-area (abdomen), or thigh.
- Inject Relistor injection exactly as your healthcare provider tells you.
- If you use Relistor injection for long-lasting (chronic) pain that is not caused by cancer:
- Inject 1 dose of Relistor injection each day.
- If you use Relistor injection and are receiving treatment for advanced illness:
- Inject 1 dose of Relistor injection every other day, as needed. You should not inject more than 1 dose of Relistor injection in a 24-hour period.
What are the possible side effects of Relistor?
See “What is the most important information I should know about Relistor?”
- The most common side effects of Relistor tablets in people with long-lasting (chronic) pain that is not caused by cancer include: stomach-area (abdomen) pain, diarrhea, headache, swelling or a feeling of fullness or pressure in your abdomen, sweating, anxiety, muscle spasms, runny nose, and chills.
- The most common side effects of Relistor injection in people with long-lasting (chronic) pain that is not caused by cancer include: stomach-area (abdomen) pain, nausea, diarrhea, sweating, hot flush, tremor, and chills.
- The most common side effects of Relistor injection in people receiving treatment for their advanced illness include: stomach-area (abdomen) pain, gas, nausea, dizziness, and diarrhea.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Relistor.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
You may also report side effects to Valeant Pharmaceuticals North America LLC at 1-800-321-4576.
General information about the safe and effective use of Relistor
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Relistor for a condition for which it was not prescribed. Do not give Relistor to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Relistor that is written for health professionals.
How should I store Relistor?
Relistor tablets:
- Store Relistor tablets at room temperature between 68° to 77°F (20° to 25°C).
- The bottle of Relistor tablets contains 2 desiccant canisters to help keep your medicine dry. Do not remove the desiccant canisters from the bottle.
Relistor injection (Vials and Pre-filled Syringes):
- Store Relistor vials and pre-filled syringes at room temperature between 68° to 77°F (20° to 25°C).
- Do not freeze Relistor vials or pre-filled syringes.
- Keep Relistor vials and pre-filled syringes away from light until you are ready to use them.
- If the contents of a Relistor vial have been drawn into a syringe and you are not able to use the medicine right away, keep the syringe at room temperature for up to 24 hours.
Keep Relistor and all medicines, needles and syringes out of the reach of children.
What are the ingredients in Relistor?
Active ingredient: methylnaltrexone bromide
Inactive ingredients:
Tablets: silicified microcrystalline cellulose, microcrystalline cellulose, sodium lauryl sulfate, croscarmellose sodium, crospovidone, poloxamer 407, stearic acid (vegetable source), colloidal silicon dioxide, edetate calcium disodium, polyvinyl alcohol, titanium dioxide, polyethylene glycol and talc
Injection: vials and pre-filled syringes: sodium chloride USP, edetate calcium disodium USP, glycine hydrochloride. During manufacture, the pH may have been adjusted with hydrochloric acid and/or sodium hydroxide.
Label
PRINCIPAL DISPLAY PANEL – 150 MG TABLETS
- NDC 65649-150-90
- RELISTOR®
- (methylnaltrexone bromide) Tablets
- 150 mg
- Dispense the accompanying Medication Guide to each patient.
- Rx only
- 90 Tablets

SRC: NLM .