RediTrex
Generic name: methotrexate (injection route, subcutaneous route)
Medically reviewed by A Ras MD.
What is RediTrex?
RediTrex is a single dose prefilled syringe containing a prescription medicine, methotrexate. Methotrexate is used to treat certain adults with severe, active rheumatoid arthritis (RA), and children with active polyarticular juvenile idiopathic arthritis (pJIA), after treatment with other medicines including non-steroidal anti-inflammatory drugs (NSAIDS) have been used and did not work well.
It is also used to control the symptoms of severe, resistant, disabling psoriasis in adults when other types of treatment have been used and did not work well.
RediTrex is available in doses of 7.5, 10, 12.5, 15, 17.5, 20, 22.5 and 25 mg. Your doctor will prescribe a different way to take methotrexate if you need to take methotrexate by mouth or in some other way. Your doctor may also change your prescription if your dose does not match the available RediTrex doses, such as doses less than 7.5 mg, or more than 25 mg, or doses between the available RediTrex doses.
RediTrex should not be used for the treatment of cancer.
RediTrex should not be used for the treatment of children with psoriasis.
Description
RediTrex contains methotrexate, a folate analog metabolic inhibitor.
Chemically, methotrexate is [N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-Lglutamic acid. The structural formula is:

C20H22N8O5
M.W. = 454.45
RediTrex contains methotrexate in a sterile, preservative-free, solution in a pre-filled syringe (in a needle safety device) with a 29 gauge ½ inch needle for a single subcutaneous injection. RediTrex solution is yellow in color.
Inactive ingredients include sodium chloride, sodium hydroxide and water for injection, USP. Sodium chloride is added to adjust tonicity. Sodium hydroxide is added to adjust pH to a target pH of 8.2.
Mechanism of Action
Methotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. Therefore, methotrexate interferes with DNA synthesis, repair, and cellular replication. Actively proliferating tissues such as malignant cells, bone marrow, fetal cells, buccal and intestinal mucosa, and cells of the urinary bladder are in general more sensitive to this effect of methotrexate.
The mechanism of action in rheumatoid arthritis is unknown; it may affect immune function.
What is the most important information I should know about RediTrex?
RediTrex can cause serious side effects that can lead to death, including:
1. Organ system toxicity. People who use methotrexate for the treatment of cancer, psoriasis, or rheumatoid arthritis, have an increased risk of death from organ toxicity. Types of organ toxicity can include:
- gastrointestinal
- bone marrow
- liver
- immune system
- nerve
- lung
- kidneys
- skin
Your doctor will do blood tests and other types of tests before you use and while you are using RediTrex to check for signs and symptoms of organ toxicity. Call your doctor right away if you have any of the following symptoms of organ toxicity.
- vomiting
- diarrhea
- mouth sores
- fever
- confusion
- weakness
- temporary blindness
- seizures
- headache
- back pain
- neck stiffness
- paralysis
- irritability
- sleepiness
- problems with coordination
- dry cough
- trouble breathing
- severe skin rash
2. Women who are pregnant are at increased risk for death of the baby and birth defects. Women who are pregnant or who plan to become pregnant must not use RediTrex. A pregnancy test should be performed before women start using RediTrex.
Contraception should be used by both females and males while using RediTrex.
Pregnancy should be avoided if either partner is using RediTrex:
- during and for a minimum of 3 months after treatment with RediTrex for males.
- during and for at least 1 menstrual cycle after treatment with RediTrex for females.
Who should not take RediTrex?
Do not use RediTrex if you:
- are pregnant or planning to become pregnant. See “What is the most important information I should know about RediTrex?”
- are breastfeeding. Methotrexate can pass into your breastmilk and may harm your baby. Do not breastfeed while using RediTrex. Talk to your doctor about the best way to feed your baby if you use RediTrex.
- have alcohol problems (alcoholism).
- have liver problems.
- have problems fighting infection (immunodeficiency syndrome).
- have been told you have (or think you have) a blood disorder such as low levels of white blood cells, red blood cells (anemia), or platelets.
- have had an allergy to methotrexate or any of the ingredients in RediTrex. See the end of this guide for a complete list of ingredients in RediTrex.
Talk to your doctor before taking this medicine if you have any of these conditions.
What should I tell my healthcare provider before taking RediTrex?
Before you use RediTrex, tell your doctor if you have any other medical conditions.
Tell your doctor about all of the medicines you take, including prescription, over-the-counter medicines, vitamins, and herbal supplements.
RediTrex may affect how other medicines work, and other medicines may affect how RediTrex works causing side effects.
Ask your doctor or pharmacist for a list of medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take RediTrex?
- Read the Instructions for use that come with RediTrex.
- Use RediTrex exactly as your doctor tells you to use it.
- Inject RediTrex only 1 time each week. Do not use RediTrex every day.
- Using RediTrex every day may cause death from toxicity.
- Your doctor will show you or your caregiver how to inject RediTrex. You should not inject RediTrex until you have been trained on the right way to use it.
- Check RediTrex before you inject it. RediTrex should be yellow in color and should not have any lumps or particles in it.
- RediTrex should be injected in the stomach (abdomen) or thigh.
- Do not inject RediTrex within 2 inches of the belly button (navel).
- Do not inject RediTrex in the arms or any other areas of the body.
- Do not inject RediTrex in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.
- If you are not sure if RediTrex was injected, or if you have a hard time giving the injection, do not inject another dose. Call your pharmacist or doctor right away.
- If you inject too much RediTrex, call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking RediTrex?
- Do not drink alcohol while using RediTrex. Drinking alcohol can increase your chances of getting serious side effects.
- RediTrex can cause dizziness and tiredness. Do not drive a car, operate machinery, or do anything that needs you to be alert until you know how RediTrex affects you.
- Certain vaccinations should be avoided while using RediTrex. Talk to your doctor before you or members of your household receive any vaccines.
What are the possible side effects of RediTrex?
RediTrex may cause serious side effects, including:
- See “What is the most important information I should know about RediTrex?”
- fertility problems. Methotrexate, the active ingredient in RediTrex may affect your ability to have a baby. Males may have a decreased sperm count, and females may have changes to their menstrual cycle. This can happen while using RediTrex and for a short period of time after you stop.
- certain cancers. Some people who have taken methotrexate have had a certain type of cancer called Non-Hodgkin’s lymphoma and other tumors. Your doctor may tell you to stop using RediTrex if this happens.
- tissue and bone problems. Taking methotrexate while having radiation therapy may increase the risk of your tissue or bone not receiving enough blood. This may lead to death of the tissue or bone.
Common side effects of RediTrex include:
- nausea
- stomach pain
- indigestion (dyspepsia)
- mouth sores
- rash
- burning skin lesions
- stuffy or runny nose and sore throat
- diarrhea
- abnormal liver function tests
- vomiting
- headache
- lung problems
- bronchitis
- low red, white, and platelet blood cell count
- hair loss
- dizziness
- sensitivity to light
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of RediTrex. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of RediTrex
Methotrexate is sometimes prescribed for purposes other than those listed in Patient Information guide. Do not use RediTrex for a condition for which it was not prescribed. Do not give RediTrex to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your doctor or pharmacist for information about RediTrex that is written for health professionals.
How should I store RediTrex?
- Store RediTrex at room temperature between 68˚F to 77˚F (20˚C to 25˚C).
- Keep RediTrex syringe in the carton until ready to use to protect from light.
Keep RediTrex and all medicines out of the reach of children.
How should I throw away (dispose) of RediTrex?
- Do not throw away in the household trash. Put used RediTrex syringes in a FDA-cleared sharps disposal container right away after use.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
What are the ingredients in RediTrex?
Active ingredient: methotrexate
Inactive ingredients: sodium chloride, sodium hydroxide, and water for injections, USP.
For more information go to www.REDITREX.com or call (877) 484-2700.
Instructions for use for RediTrex
RediTrexTM (re-dee-treks)
(methotrexate injection, for subcutaneous use)
Important: Do not share your syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Use these instructions with all doses of RediTrex. Make sure that you have the right dose that has been prescribed for you.
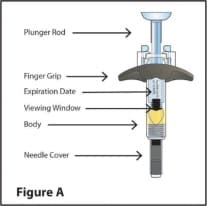
RediTrex Prefilled Syringe Parts (See Figure A)
Note: The plunger rod for your dose may be a different color than shown in the Figures in this Instructions for Use.
Supplies needed to give your injection (See Figure B)
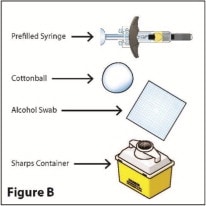
Only the prefilled syringe is included in the package
- A clean flat well-lit surface, such as a table
- 1 dose tray containing a RediTrex prefilled syringe with a fixed needle
- 1 alcohol prep (swab)
- 1 cotton ball or gauze pad
- 1 puncture-resistant sharps container for safe disposal of used needles and syringes. (See Step 8, “How Should I Dispose of Used Prefilled Syringes and Needles?”)
Make sure you have all of the items you need to give yourself an injection.
Step 1. Prepare to use RediTrex
- Do not remove needle cover until you are ready to inject RediTrex.
- Check the expiration date on the label of prefilled syringe. (See Figure C)

- Do not use if expired. (See Step 8)
- Wash your hands well with soap and warm water.
Step 2. Check the liquid
- The liquid in the syringe should be yellow in color and should not have any lumps or particles in it.
- You may see air bubbles. This is normal.
Step 3. Choose an injection site
- RediTrex should be injected into the stomach (abdomen) or thigh. (See Figure D)
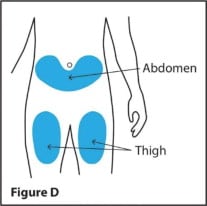
- Do not inject RediTrex within 2 inches of the belly button (navel).
- Do not inject RediTrex in the arms or any other areas of the body.
- Do not inject RediTrex in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.
Step 4. Clean the injection site
- Wipe the area with an alcohol (prep) swab. (See Figure E)
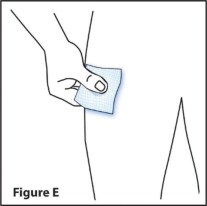
- Let the skin dry. Do not touch this area again before giving RediTrex.
- Do not fan or blow the clean area.
Step 5. Prepare the syringe and needle
- Always hold the prefilled syringe by the body of the syringe.
- Remove needle cover.
- Hold the syringe in 1 hand. With the other hand gently remove the needle cover by pulling it straight off. (See Figure F). Do not hold or touch the plunger while you remove the needle cover.
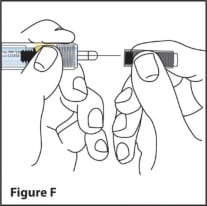
- Throw away the needle cover in a puncture-resistant sharps container right away. (See Step 8)
- Do not touch the needle with your fingers or let the needle touch anything.
- You may see a drop of liquid at the end of the needle. This is normal.
Step 6. Inject RediTrex
- Hold the body of the prefilled syringe in 1 hand between the thumb and index finger. Hold the syringe in your hand like a pencil. (See Figure G)
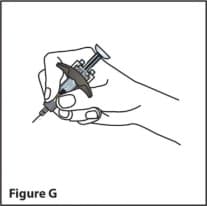
- Do Not pull back on the plunger at any time.
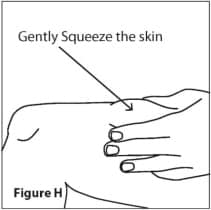
- With your other hand, gently squeeze the area of the cleaned skin and hold it firmly. (See Figure H)
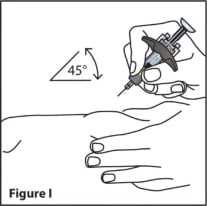
- Using a quick, dart-like motion, insert the needle into the squeezed skin at about a 45-degree angle. (See Figure I)
- Slowly push the plunger all the way in until all of the liquid is injected and the syringe is empty. (See Figure J)
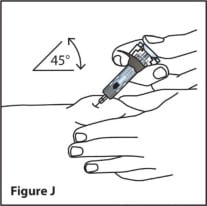
- When the plunger is pushed all the way in, the needle withdraws automatically into the body of the syringe and the needle will be automatically covered.
Step 7. After the injection
- Press a cotton ball or gauze pad over the injection site and hold it for 10 seconds. Do not rub the injection site. You may have a small amount of bleeding. This is normal.
- Throw away the used prefilled syringe and needle. See Step 8 (“How Should I Dispose of Used Prefilled Syringes and Needles?”)
- Keep a record of the dates and location of your injection sites. To help you remember when to take RediTrex, you can mark your calendar ahead of time.
Step 8. How should I dispose of used needle and syringe?
- Put your used needle and syringe in a FDA-cleared sharps disposal container right away after use (See Figure K). Do not throw away (dispose of) loose needles and syringes in your household trash.
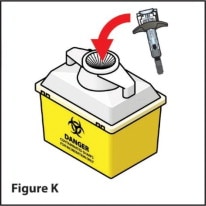
- Do not try to touch the needle.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http:// www.fda.gov/safesharpsdisposal.
- For the safety and health of you and others, needles and used syringes must never be re-used.
- The used alcohol pads, cotton balls, dose trays and packaging may be placed in your household trash.
- Do not dispose of your used sharps disposal container in your household trash unless your com- munity guidelines permit this. Do not recycle your used sharps disposal container.
- Always keep the sharps container out of the reach of children.
How should I store RediTrex?
Store RediTrex at room temperature between 68°F to 77°F (20°C to 25°C).
Keep RediTrex in the carton until ready to use to protect from light.
Label
Principal Display Panel – 12.5 mg/0.5 mL Carton Label
- NDC 66220-812-11
- RediTrex™ 12.5 mg per 0.5 mL Rx only
- (methotrexate) injection
- This carton contains 1 prefilled syringe.
- Each prefilled syringe of 0.5 mL contains 12.5 mg methotrexate in a
sterile solution. - Inactive ingredients include sodium chloride and water for injection, USP.
- Sodium hydrochloride is added to adjust pH.
- See full prescribing information for dosage and administration.
- Read enclosed patient package insert carefully before using.
CUMBERLAND®
PHARMACEUTICALS

Principal Display Panel – 15 mg/0.6 mL Case Label
- NDC 66220-815-22
- 15 mg
- RediTrex™ 15 mg per 0.6 mL Rx only
- (methotrexate) injection
- FOR SUBCUTANEOUS USE ONLY
- Each syringe is for one single dose only.
- Use once weekly.
- This box contains 4 prefilled single use RediTrex syringes.
- See full prescribing information for dosage and administration.
- Read enclosed patient package insert carefully before using.
CUMBERLAND®
PHARMACEUTICALS

SRC: NLM .
