Promacta
Generic name: eltrombopag
Drug class: Platelet-stimulating agents
Medically reviewed by A Ras MD.
What is Promacta?
Promacta is a prescription medicine used to treat adults and children 1 year of age and older with low blood platelet counts due to chronic immune thrombocytopenia (ITP), when other medicines to treat ITP or surgery to remove the spleen have not worked well enough.
Promacta is also used to treat people with:
- low blood platelet counts due to chronic hepatitis C virus (HCV) infection before and during treatment with interferon.
- severe aplastic anemia (SAA) in combination with other medicines to treat SAA, as the first treatment for adults and children 2 years of age and older.
- severe aplastic anemia (SAA) when other medicines to treat SAA have not worked well enough.
Promacta is used to try to raise platelet counts in order to lower your risk for bleeding.
Promacta is not used to make platelet counts normal.
Promacta is not for use in people with a pre-cancerous condition called myelodysplastic syndrome (MDS), or in people with low platelet counts caused by certain other medical conditions or diseases.
It is not known if Promacta is safe and effective when used with other antiviral medicines to treat chronic hepatitis C.
It is not known if Promacta is safe and effective in children:
- younger than 1 year with ITP
- with low blood platelet counts due to chronic hepatitis C
- whose severe aplastic anemia (SAA) has not improved after previous treatments.
- younger than 2 years when used in combination with other medicines to treat SAA as the first treatment for SAA.
Description
PROMACTA (eltrombopag) tablets contain eltrombopag olamine, a small molecule thrombopoietin (TPO) receptor agonist for oral administration. Eltrombopag interacts with the transmembrane domain of the TPO receptor (also known as cMpl) leading to increased platelet production.
Eltrombopag olamine is a biphenyl hydrazone. The chemical name for eltrombopag olamine is 3′-{(2Z)-2-[1-(3,4-dimethylphenyl)-3-methyl-5-oxo-1,5-dihydro-4H-pyrazol-4-ylidene]hydrazino}-2′-hydroxy-3-biphenylcarboxylic acid – 2-aminoethanol (1:2). It has the molecular formula C25H22N4O4 • 2(C2H7NO). The molecular weight is 564.65 g/mol for eltrombopag olamine and 442.5 g/mol for eltrombopag free acid. Eltrombopag olamine has the following structural formula:
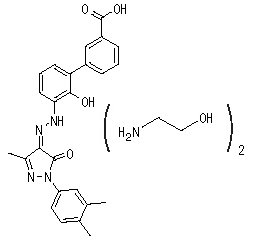
Eltrombopag olamine is practically insoluble in aqueous buffer across a pH range of 1 to 7.4, and is sparingly soluble in water.
PROMACTA (eltrombopag) tablets contain eltrombopag olamine in the amount equivalent to 12.5 mg, 25 mg, 50 mg, or 75 mg of eltrombopag free acid. The inactive ingredients of PROMACTA tablets are:
Tablet Core: magnesium stearate, mannitol, microcrystalline cellulose, povidone, and sodium starch glycolate.
Coating: FD&C Blue No. 2 aluminum lake (50-mg tablet), FD&C Yellow No. 6 aluminum lake (25-mg tablet), hypromellose, Iron Oxide Black and Iron Oxide Red (75-mg tablet), polyethylene glycol 400, polysorbate 80 (12.5-mg tablet), or titanium dioxide.
PROMACTA (eltrombopag) for oral suspension packets contain a reddish-brown to yellow powder which produces a reddish-brown suspension when reconstituted with water. Each packet delivers eltrombopag olamine equivalent to 12.5 mg or 25 mg of eltrombopag free acid. The inactive ingredients of PROMACTA for oral suspension are mannitol, sucralose, and xanthan gum.
Mechanism of Action
Eltrombopag is an orally bioavailable, small-molecule TPO-receptor agonist that interacts with the transmembrane domain of the human TPO-receptor and initiates signaling cascades that induce proliferation and differentiation from bone marrow progenitor cells.
What is the most important information I should know about Promacta?
Promacta can cause serious side effects, including:
Liver problems:
- If you have chronic hepatitis C virus and take Promacta with interferon and ribavirin treatment, Promacta may increase your risk of liver problems. If your healthcare provider tells you to stop your treatment with interferon and ribavirin, you will also need to stop taking Promacta.
- Promacta may increase your risk of liver problems that may be severe and possibly life threatening. Your healthcare provider will do blood tests to check your liver function before you start taking Promacta and during your treatment. Your healthcare provider may stop your treatment with Promacta if you have changes in your liver function blood tests.
Tell your healthcare provider right away if you have any of these signs and symptoms of liver problems:
- yellowing of the skin or the whites of the eyes (jaundice)
- unusual darkening of the urine
- unusual tiredness
- right upper stomach area (abdomen) pain
- confusion
- swelling of the stomach area (abdomen)
See “What are the possible side effects of Promacta?” for other side effects of Promacta.
What should I tell my healthcare provider before taking Promacta?
Before you take Promacta, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems
- have a precancerous condition called MDS or a blood cancer
- have or had a blood clot
- have a history of cataracts
- have had surgery to remove your spleen (splenectomy)
- have bleeding problems
- are of Asian ancestry (such as Chinese, Japanese, Taiwanese, or Korean). You may need a lower dose of Promacta.
- are pregnant or plan to become pregnant. It is not known if Promacta will harm an unborn baby. Tell your healthcare provider if you become pregnant or think you may be pregnant during treatment with Promacta.
- Females who are able to become pregnant, should use effective birth control (contraception) during treatment with Promacta and for at least 7 days after stopping treatment with Promacta. Talk to your healthcare provider about birth control methods that may be right for you during this time.
- are breastfeeding or plan to breastfeed. You should not breastfeed during your treatment with Promacta. Talk to your healthcare provider about the best way to feed your baby during this time.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Promacta may affect the way certain medicines work. Certain other medicines may affect the way Promacta works.
Especially tell your healthcare provider if you take:
- certain medicines used to treat high cholesterol, called “statins”
- a blood thinner medicine
Certain medicines may keep Promacta from working correctly. Take Promacta at least 2 hours before or 4 hours after taking these products:
- antacid medicine used to treat stomach ulcers or heartburn
- multivitamins or products that contain iron, calcium, aluminum, magnesium, selenium, and zinc which may be found in mineral supplements
Ask your healthcare provider if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Promacta?
- Take Promacta exactly as your healthcare provider tells you to take it. Your healthcare provider will prescribe the dose of Promacta tablets or Promacta for oral suspension that is right for you.
- If your healthcare provider prescribes Promacta tablets, take Promacta tablets whole. Do not split, chew, or crush Promacta tablets and do not mix with food or liquids.
- If your healthcare provider prescribes Promacta for oral suspension, see the “Instructions for Use” that comes with your medicine for instructions on how to correctly mix and take a dose of Promacta.
- Use a new single-use oral dosing syringe to prepare each dose of Promacta for oral suspension. Do not re-use the oral dosing syringe.
- Do not stop taking Promacta without talking with your healthcare provider first. Do not change your dose or schedule for taking Promacta unless your healthcare provider tells you to change it.
- Take Promacta on an empty stomach, either 1 hour before or 2 hours after eating food.
- Take Promacta at least 2 hours before or 4 hours after eating dairy products and calcium-fortified juices.
- If you miss a dose of Promacta, wait and take your next scheduled dose. Do not take more than 1 dose of Promacta in 1 day.
- If you take too much Promacta, you may have a higher risk of serious side effects. Call your healthcare provider right away.
- Your healthcare provider will check your platelet count during your treatment with Promacta and change your dose of Promacta as needed.
- Tell your healthcare provider about any bruising or bleeding that happens while you take and after you stop taking Promacta.
- If you have SAA, your healthcare provider may do tests to monitor your bone marrow during treatment with Promacta.
What should I avoid while taking Promacta?
Avoid situations and medicines that may increase your risk of bleeding.
What are the possible side effects of Promacta?
Promacta may cause serious side effects, including:
- See “What is the most important information I should know about Promacta?”
- Increased risk of worsening of a precancerous blood condition called myelodysplastic syndrome (MDS) to acute myelogenous leukemia (AML). Promacta is not for use in people with a precancerous condition called myelodysplastic syndromes (MDS). See “What is Promacta?” If you have MDS and receive Promacta, you have an increased risk that your MDS condition may worsen and become a blood cancer called AML. If your MDS worsens to become AML, you may have an increased risk of death from AML.
- High platelet counts and higher risk for blood clots. Your risk of getting a blood clot is increased if your platelet count is too high during treatment with Promacta. Your risk of getting a blood clot may also be increased during treatment with Promacta if you have normal or low platelet counts. You may have severe problems or die from some forms of blood clots, such as clots that travel to the lungs or that cause heart attacks or strokes. Your healthcare provider will check your blood platelet counts, and change your dose or stop Promacta if your platelet counts get too high. Tell your healthcare provider right away if you have signs and symptoms of a blood clot in the leg, such as swelling, pain, or tenderness in your leg.
People with chronic liver disease may be at risk for a type of blood clot in the stomach area (abdomen). Tell your healthcare provider right away if you have stomach-area (abdomen) pain, nausea, vomiting, or diarrhea as these may be symptoms of this type of blood clot. - New or worsened cataracts (a clouding of the lens in the eye). New or worsened cataracts can happen in people taking Promacta. Your healthcare provider will check your eyes before and during your treatment with Promacta. Tell your healthcare provider about any changes in your eyesight while taking Promacta.
The most common side effects of Promacta in adults and children include:
- low red blood cell count (anemia)
- nausea
- fever
- abnormal liver function tests
- cough
- tiredness
- headache
- diarrhea
Laboratory tests may show abnormal changes to the cells in your bone marrow.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Promacta. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Promacta
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Promacta for a condition for which it was not prescribed. Do not give Promacta to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Promacta that is written for health professionals.
How should I store Promacta?
Tablets:
- Store Promacta tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Promacta in the bottle given to you.
For oral suspension:
- Store Promacta for oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- After mixing, Promacta should be taken right away but may be stored for no more than 30 minutes between 68°F to 77°F (20°C to 25°C). Throw away (discard) the mixture if not used within 30 minutes.
Keep Promacta and all medicines out of the reach of children.
What are the ingredients in Promacta?
Tablets
Active ingredient: eltrombopag olamine
Inactive ingredients:
- Tablet Core: magnesium stearate, mannitol, microcrystalline cellulose, povidone, and sodium starch glycolate.
- Coating: FD&C Blue No. 2 aluminum lake (50-mg tablet), FD&C Yellow No. 6 aluminum lake (25-mg tablet), hypromellose, Iron Oxide Black (75-mg tablet) and Iron Oxide Red (75-mg tablet), polyethylene glycol 400, polysorbate 80 (12.5-mg tablet), or titanium dioxide.
For oral suspension
Active ingredient: eltrombopag olamine.
Inactive ingredients: mannitol, sucralose, xanthan gum
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-0685-15
- Rx only
- PROMACTA®
- (eltrombopag) Tablets
- 25 mg*
- *Each tablet contains eltrombopag olamine equivalent to 25 mg of eltrombopag free acid.
- Dispense with Medication Guide attached or provided separately.
- NOVARTIS
- 30 Tablets


PRINCIPAL DISPLAY PANEL
- NDC 0078-0686-15
- Rx only
- PROMACTA®
- (eltrombopag) Tablets
- 50 mg*
- *Each tablet contains eltrombopag olamine equivalent to 50 mg of eltrombopag free acid.
- Dispense with Medication Guide attached or provided separately.
- NOVARTIS
- 30 Tablets


PRINCIPAL DISPLAY PANEL
- NDC 0078-0687-15
- Rx only
- PROMACTA®
- (eltrombopag) Tablets
- 75 mg*
- *Each tablet contains eltrombopag olamine equivalent to 75 mg of eltrombopag free acid.
- Dispense with Medication Guide attached or provided separately.
- NOVARTIS
- 30 Tablets

PRINCIPAL DISPLAY PANEL
- NDC 0078-0972-61
- Rx only
- PROMACTA®
- (eltrombopag)
for Oral Suspension - 12.5 mg
- Dispense with Medication Guide enclosed or provided separately
- 30 Packets
- Novartis

SRC: NLM .