Prezcobix
Generic name: cobicistat and darunavir
Drug class: Antiviral combinations
Medically reviewed by A Ras MD.
What is Prezcobix?
Prezcobix is a prescription HIV-1 (Human Immunodeficiency Virus 1) medicine used with other antiretroviral medicines to treat HIV-1 infection in adults. HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
Prezcobix contains the prescription medicines darunavir and cobicistat.
It is not known if Prezcobix is safe and effective in children under 18 years of age.
Description
PREZCOBIX® is a fixed-dose combination tablet containing darunavir and cobicistat. Darunavir is an inhibitor of the human immunodeficiency virus (HIV-1) protease. Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
PREZCOBIX tablets are for oral administration. Each tablet contains darunavir ethanolate equivalent to 800 mg of darunavir and 150 mg of cobicistat. The tablets include the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, and silicified microcrystalline cellulose. The tablets are film-coated with a coating material containing iron oxide black, iron oxide red, polyethylene glycol, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
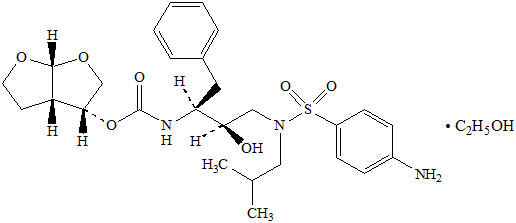
Darunavir: Darunavir, in the form of darunavir ethanolate, has the following chemical name: [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-carbamic acid (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester monoethanolate. Its molecular formula is C27H37N3O7S ∙ C2H5OH and its molecular weight is 593.73. Darunavir ethanolate has the following structural formula:
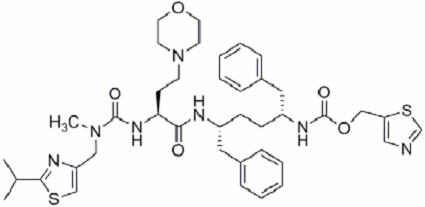
Cobicistat: Cobicistat is adsorbed onto silicon dioxide. The chemical name for cobicistat is 1,3-thiazol-5-ylmethyl[(2R,5R)-5-{[(2S)2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate. It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.0. It has the following structural formula:
Mechanism of Action
PREZCOBIX is a fixed-dose combination of an HIV-1 antiviral drug, darunavir and a CYP3A inhibitor, cobicistat
What is the most important information I should know about Prezcobix?
- Prezcobix may cause liver problems. Some people taking Prezcobix may develop liver problems which may be life-threatening. Your healthcare provider should do blood tests before and during your treatment with Prezcobix. If you have chronic hepatitis B or C infection, your healthcare provider should check your blood tests more often because you have an increased chance of developing liver problems. Tell your healthcare provider if you have any of the below signs and symptoms of liver problems.
- Prezcobix may cause severe or life-threatening skin reactions or rash. Sometimes these skin reactions and skin rashes can become severe and require treatment in a hospital. Call your healthcare provider right away if you develop a rash. Stop taking Prezcobix and call your healthcare provider right away if you develop any skin changes with symptoms below:
- fever
- tiredness
- muscle or joint pain
- blisters or skin lesions
- mouth sores or ulcers
- red or inflamed eyes, like “pink eye” (conjunctivitis)
- Prezcobix when taken with certain other medicines can cause new or worse kidney problems, including kidney failure. Your healthcare provider should check your kidneys before you start and while you are taking Prezcobix.
See “What are the possible side effects of Prezcobix?” for more information about side effects.
Who should not take Prezcobix?
Do not take Prezcobix with any medicine that contains:
- alfuzosin
- carbamazepine
- cisapride
- colchicine, if you have liver or kidney problems
- dronedarone
- elbasvir and grazoprevir
- ergot-containing medicines:
- dihydroergotamine
- ergotamine tartrate
- methylergonovine
- ivabradine
- lomitapide
- lovastatin
- lurasidone
- midazolam, when taken by mouth
- naloxegol
- phenobarbital
- phenytoin
- pimozide
- ranolazine
- rifampin
- sildenafil, when used for the treatment of pulmonary arterial hypertension (PAH)
- simvastatin
- St. John’s wort (Hypericum perforatum)
- triazolam
Serious problems can happen if you take any of these medicines with Prezcobix.
What should I tell my healthcare provider before taking Prezcobix?
Before taking Prezcobix, tell your healthcare provider about all your medical conditions, including if you:
- have liver problems, including hepatitis B or hepatitis C
- have kidney problems
- are allergic to sulfa (sulfonamide)
- have diabetes
- have hemophilia
- have any other medical condition
- are pregnant or plan to become pregnant.
- It is not known if Prezcobix will harm your unborn baby.
- Prezcobix should not be used in pregnant individuals because you may not have enough Prezcobix in your body during pregnancy.
- Tell your healthcare provider if you become pregnant while taking Prezcobix. Your healthcare provider will prescribe different medicines if you become pregnant while taking Prezcobix.
- Pregnancy Registry: There is a pregnancy registry for individuals who take antiretroviral medicines during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Prezcobix.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV to your baby.
- It is not known if Prezcobix can pass into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with Prezcobix. Keep a list of your medicines to show your healthcare provider and pharmacist.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Prezcobix.
- Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Prezcobix with other medicines.
How should I take Prezcobix?
- Take Prezcobix exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Prezcobix without talking to your healthcare provider.
- Take Prezcobix 1 time a day with food.
- Do not miss a dose of Prezcobix.
- If you take too much Prezcobix, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Prezcobix?
Prezcobix may cause serious side effects, including:
- See “What is the most important information I should know about Prezcobix?”
- Diabetes and high blood sugar (hyperglycemia). Some people who take protease inhibitors including Prezcobix can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or urinate often while taking Prezcobix.
- Changes in body fat can happen in people who take HIV-1 medications. The changes may include an increased amount of fat in the upper back and neck (“buffalo hump”), breast, and around the middle of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these conditions are not known.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- Increased bleeding for hemophiliacs. Some people with hemophilia have increased bleeding with protease inhibitors including Prezcobix.
The most common side effects of darunavir, one of the medicines in Prezcobix, include:
These are not all of the possible side effects of Prezcobix.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Prezcobix
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Prezcobix for a condition for which it was not prescribed. Do not give Prezcobix to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Prezcobix that is written for health professionals.
How should I store Prezcobix?
- Store Prezcobix tablets at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Prezcobix and all medicines out of reach of children.
What are the ingredients in Prezcobix?
Active ingredients: darunavir and cobicistat
Inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, and silicified microcrystalline cellulose. The tablets are film-coated with a coating material containing iron oxide black, iron oxide red, polyethylene glycol, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 800 MG/150 MG TABLET BOTTLE LABEL
- NDC 59676-575-30
- PREZCOBIX®
(darunavir and cobicistat)
Tablets - 800 mg/150 mg
- Each tablet contains darunavir
ethanolate (equivalent to
800 mg of darunavir) and
150 mg cobicistat. - Keep out of reach of children
- Rx only
30 Tablets

SRC: NLM .
