Pancreaze
Generic name: pancrelipase
Drug class: Digestive enzymes
Medically reviewed by A Ras MD.
What is Pancreaze?
Pancreaze is a prescription medicine used to treat people who cannot digest food normally because their pancreas does not make enough enzymes due to cystic fibrosis or other conditions. Pancreaze may help your body use fats, proteins, and sugars from food.
Pancreaze contains a mixture of digestive enzymes including lipases, proteases, and amylases from pig pancreas.
Pancreaze is safe and effective in children when taken as prescribed by your doctor.
Description
Pancrelipase is a pancreatic enzyme preparation consisting of an extract derived from porcine pancreatic glands. Pancrelipase contains multiple enzyme classes, including porcine-derived lipases, proteases, and amylases. The minimum potency in each mg of pancrelipase, as described per USP, is not less than 24 units of lipase activity, not less than 100 units of amylase activity, and not less than 100 units of protease activity.
Each PANCREAZE (pancrelipase) delayed-release capsule for oral administration contains enteric-coated microtablets that are each approximately 2 mm in diameter.
The active ingredient evaluated in clinical trials is lipase. PANCREAZE is dosed by lipase units. Other active ingredients include protease and amylase.
Inactive ingredients in all PANCREAZE strengths include colloidal silicon dioxide, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, microcrystalline cellulose, montan glycol wax, simethicone emulsion, talc and triethyl citrate.
PANCREAZE is available in five color coded strengths. Each PANCREAZE delayed-release capsule strength contains the specified amounts of lipase, protease, and amylase as follows:
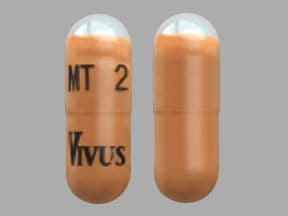
2,600 USP units of lipase; 8,800 USP units of protease; 15,200 USP units of amylase. The hypromellose capsules have a light orange opaque body and clear cap imprinted with “VIVUS” and “MT 2”. The capsule shell contains hypromellose, titanium dioxide, yellow iron oxide, red iron oxide, and imprint ink contains black iron oxide, shellac, propylene glycol, strong ammonia solution, potassium hydroxide.
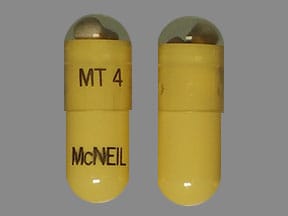
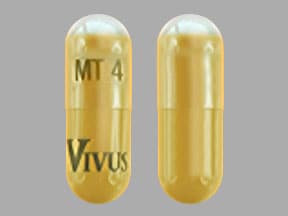
4,200 USP units of lipase; 14,200 USP units of protease; 24,600 USP units of amylase. The hypromellose capsules have a yellow opaque body and clear cap imprinted with “VIVUS” and “MT 4”. The capsule shell contains hypromellose, titanium dioxide, yellow iron oxide, and imprint ink contains black iron oxide, shellac-glaze-45%, ammonium hydroxide, propylene glycol..
10,500 USP units of lipase; 35,500 USP units of protease; 61,500 USP units of amylase. The hypromellose capsules have a flesh opaque body and clear cap imprinted with “VIVUS” and “MT 10”. The capsule shell contains hypromellose, titanium dioxide, red iron oxide, and imprint ink contains black iron oxide, shellac-glaze-45%, ammonium hydroxide, propylene glycol.
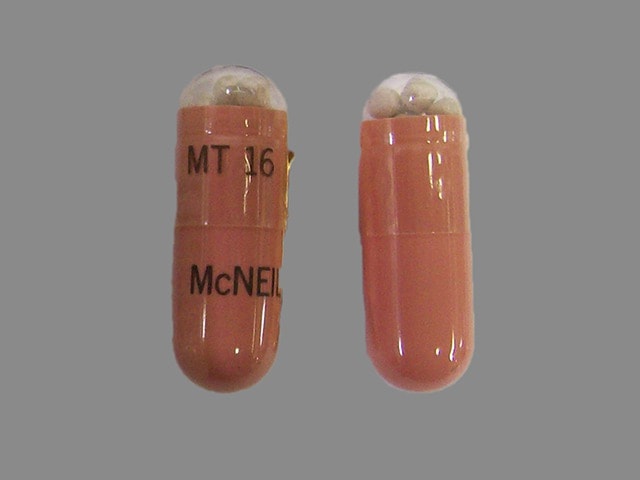
16,800 USP units of lipase; 56,800 USP units of protease; 98,400 USP units of amylase. The hypromellose capsules have a flesh opaque body and clear cap imprinted with “VIVUS” and “MT 16”. The capsule shell contains hypromellose, titanium dioxide, red iron oxide, yellow iron oxide, and imprint ink contains black iron oxide, shellac-glaze-45%, ammonium hydroxide, propylene glycol.
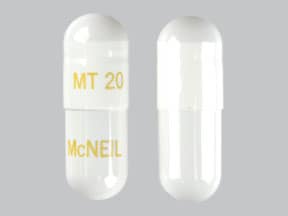
21,000 USP units of lipase; 54,700 USP units of protease; 83,900 USP units of amylase. The hypromellose capsules have a white opaque body and cap imprinted with “VIVUS” and “MT 20”. The capsule shell contains hypromellose, titanium dioxide, and imprint ink contains yellow iron oxide, shellac, strong ammonia solution, propylene glycol.
37,000 USP units of lipase; 97,300 USP units of protease; 149,900 USP units of amylase. The hypromellose capsules have an iron grey opaque body and white opaque cap imprinted with “VIVUS” and “MT 37”. The capsule shell contains hypromellose, titanium dioxide, black iron oxide and imprint ink contains black iron oxide, shellac-glaze-45%, ammonium hydroxide, propylene glycol.
Mechanism of Action
The pancreatic enzymes in PANCREAZE catalyze the hydrolysis of fats to monoglyceride, glycerol and free fatty acids, proteins into peptides and amino acids, and starches into dextrins and short chain sugars such as maltose and maltriose in the duodenum and proximal small intestine, thereby acting like digestive enzymes physiologically secreted by the pancreas.
What is the most important information I should know about Pancreaze?
Pancreaze may increase your chance of having a rare bowel disorder called fibrosing colonopathy. This condition is serious and may require surgery. The risk of having this condition may be reduced by following the dosing instructions that your doctor gave you.
Call your doctor right away if you have any unusual or severe:
- stomach area (abdominal) pain
- bloating
- trouble passing stool (having bowel movements)
- nausea, vomiting, or diarrhea
Take Pancreaze exactly as prescribed by your doctor. Do not take more or less Pancreaze than directed by your doctor.
What should I tell my healthcare provider before taking Pancreaze?
Before taking Pancreaze, tell your doctor about all your medical conditions, including if you:
- are allergic to pork (pig) products.
- have a history of blockage of your intestines, or scarring or thickening of your bowel wall (fibrosing colonopathy)
- have gout, kidney disease, or high blood uric acid (hyperuricemia)
- have trouble swallowing capsules
- have any other medical condition
- are pregnant or plan to become pregnant. It is not known if Pancreaze will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Pancreaze passes into your breast milk. You and your doctor should decide if you will take Pancreaze or breastfeed.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take Pancreaze?
Take Pancreaze exactly as your doctor tells you.
- Do not take more capsules in a day than the number your doctor tells you to take (total daily dose).
- Always take Pancreaze with a meal or snack and plenty of fluid. If you eat a lot of meals or snacks in a day, be careful not to go over your total daily dose.
- Your doctor may change your dose based on the amount of fatty foods you eat or based on your weight.
- Do not crush or chew the Pancreaze capsules or their contents, and do not hold the capsule or contents in your mouth. Crushing, chewing or holding the Pancreaze capsules in your mouth may cause irritation in your mouth or change the way Pancreaze works in your body.
Giving Pancreaze to infants (children up to 12 months):
- Give Pancreaze right before each feeding of formula or breast milk.
- Do not mix Pancreaze capsule contents directly into formula or breast milk.
- Open the capsules and sprinkle the contents directly into your infant’s mouth or mix the contents in a small amount of soft food such as applesauce. These foods should be the kind found in baby food jars that you buy at the store, or other food recommended by your doctor.
- If you sprinkle the Pancreaze on food, give the Pancreaze and food mixture to your child right away. Do not store Pancreaze that is mixed with food.
- Give your child enough liquid to completely swallow the Pancreaze contents or the Pancreaze and food mixture.
- Look into your child’s mouth to make sure that all of the medicine has been swallowed.
Giving Pancreaze to children and adults
- Swallow Pancreaze capsules whole and take them with enough liquid to swallow them right away.
- If you have trouble swallowing capsules, open the capsules and sprinkle the contents on a small amount of acidic food such as applesauce. Ask your doctor about other foods you can mix with Pancreaze.
- If you sprinkle Pancreaze on food, swallow it right after you mix it and drink plenty of water or juice to make sure the medicine is swallowed completely. Do not store Pancreaze that is mixed with food.
- If you forget to take Pancreaze, call your healthcare provider or wait until your next meal and take your usual number of capsules. Take your next dose at your usual time. Do not make up for missed doses.
What are the possible side effects of Pancreaze?
Pancreaze may cause serious side effects, including:
- See “What is the most important information I should know about Pancreaze?”
- Irritation of the inside of your mouth. This can happen if Pancreaze is not swallowed completely.
- Increase in blood uric acid levels. This may cause worsening of swollen, painful joints (gout) caused by an increase in your blood uric acid levels
- Allergic reactions including trouble with breathing, skin rashes, or swollen lips.
- Call your doctor right away if you have any of these symptoms.
The most common side effects of Pancreaze include:
- Pain in your stomach (abdominal area)
- nausea
- Gas
Other possible side effects of Pancreaze:
Pancreaze and other pancreatic enzyme products are made from the pancreas of pigs, the same pigs people eat as pork. These pigs may carry viruses. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs.
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of Pancreaze. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to VIVUS, Inc. at 1-888-998-4887.
General information about the safe and effective use of Pancreaze
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Pancreaze for a condition for which it was not prescribed. Do not give Pancreaze to other people to take, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about Pancreaze. If you would like more information, talk to your doctor. You can ask your pharmacist or doctor for information about Pancreaze that is written for healthcare professionals.
For more information go to www.pancreaze.net or call 1-888-998-4887.
How should I store Pancreaze?
- Store Pancreaze at room temperature below 77°F (25°C). Avoid heat.
- Keep Pancreaze in a dry place and in the original container.
- After opening the bottle, keep it closed tightly between uses.
- The Pancreaze 2,600 USP units of lipase, the Pancreaze 4,200 USP units of lipase and Pancreaze 21,000 USP units of lipase bottles contain a desiccant canister. Do not eat or throw away the desiccant canister in your medicine bottle. This canister will protect your medicine from moisture.
Keep Pancreaze and all medicines out of the reach of children.
What are the ingredients in Pancreaze?
Active Ingredient: lipase, protease, amylase
Inactive ingredients in Pancreaze 2,600 USP units of lipase: colloidal silicon dioxide, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, microcrystalline cellulose, montan glycol wax, simethicone emulsion, talc and triethyl citrate. The capsule shell contains hypromellose, titanium dioxide, iron oxide, and imprint ink contains iron oxide, shellac, ammonium hydroxide, propylene glycol, potassium hydroxide.
Inactive ingredients in other strengths: cellulose, colloidal anhydrous silica, crospovidone, magnesium stearate, methacrylic acid ethyl acrylate copolymer, montan glycol wax, simethicone emulsion, talc and triethyl citrate. The capsule shell contains gelatin, titanium dioxide, sodium lauryl sulfate, sorbitan monolaurate, and gelatin capsule imprint ink. Pancreaze 4,200, 10,500, and 16,800 USP units of lipase also contain iron oxide.
Label
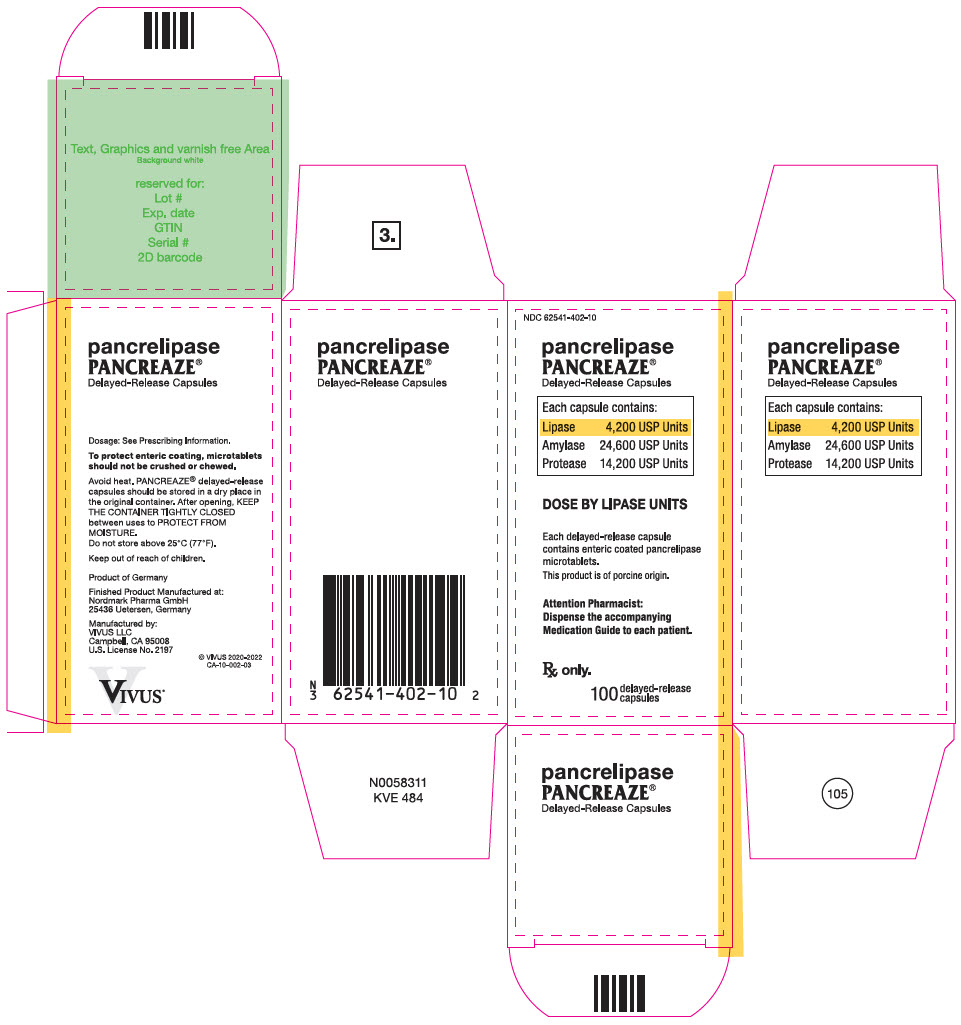
PRINCIPAL DISPLAY PANEL – 100 CAPSULE BOTTLE CARTON – NDC 62541-402-10
- NDC 62541-402-10
- pancrelipase
PANCREAZE ®
Delayed-Release Capsules - Each capsule contains:
- Lipase 4,200 USP Units
Amylase 24,600 USP Units
Protease 14,200 USP Units - DOSE BY LIPASE UNITS
- Each delayed-release capsule
contains enteric coated pancrelipase
microtablets.
This product is of porcine origin. - Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient. - Rx only.
- 100
delayed-release
capsules
PRINCIPAL DISPLAY PANEL – 100 CAPSULE BOTTLE CARTON – NDC 62541-403-10
- NDC 62541-403-10
- pancrelipase
PANCREAZE ®
Delayed-Release Capsules - Each capsule contains:
- Lipase 10,500 USP Units
Amylase 61,500 USP Units
Protease 35,500 USP Units - DOSE BY LIPASE UNITS
- Each delayed-release capsule contains
enteric coated pancrelipase microtablets.
This product is of porcine origin. - Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient. - Rx only.
- 100
delayed-release
capsules

PRINCIPAL DISPLAY PANEL – 100 CAPSULE BOTTLE CARTON – NDC 62541-404-10
- NDC 62541-404-10
- pancrelipase
PANCREAZE ®
Delayed-Release Capsules - Each capsule contains:
- Lipase 16,800 USP Units
Amylase 98,400 USP Units
Protease 56,800 USP Units - DOSE BY LIPASE UNITS
- Each delayed-release capsule contains
enteric coated pancrelipase microtablets.
This product is of porcine origin. - Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient. - Rx only.
- 100
delayed-release
capsules

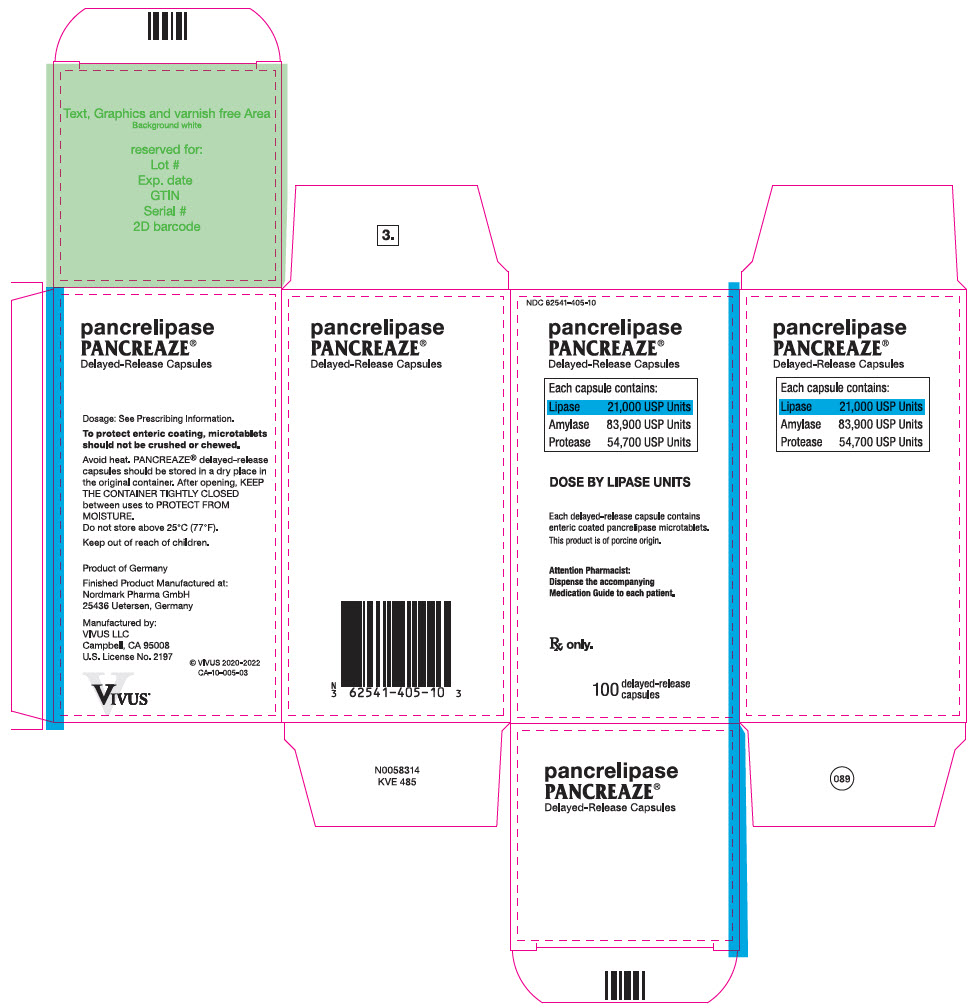
PRINCIPAL DISPLAY PANEL – 100 CAPSULE BOTTLE CARTON – NDC 62541-405-10
- NDC 62541-405-10
- pancrelipase
PANCREAZE ®
Delayed-Release Capsules - Each capsule contains:
- Lipase 21,000 USP Units
Amylase 83,900 USP Units
Protease 54,700 USP Units - DOSE BY LIPASE UNITS
- Each delayed-release capsule contains
enteric coated pancrelipase microtablets.
This product is of porcine origin. - Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient. - Rx only.
- 100
delayed-release
capsules
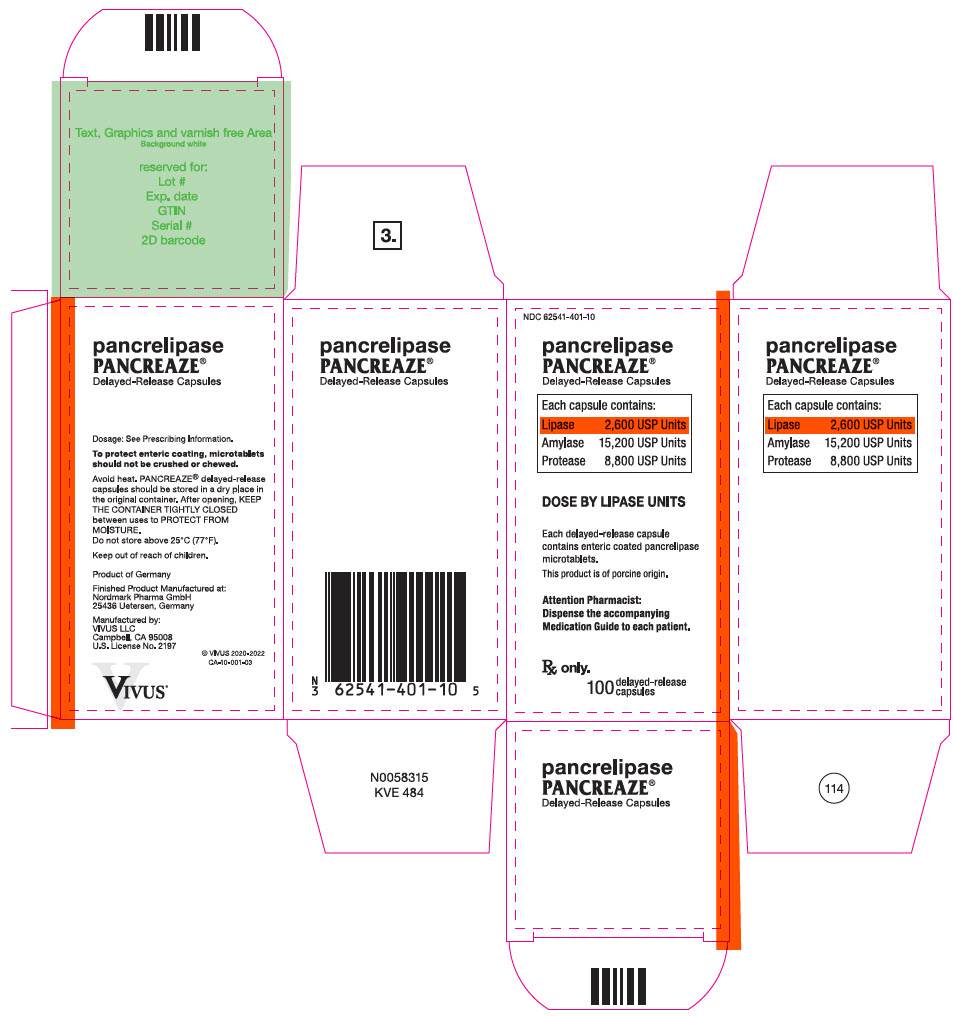
PRINCIPAL DISPLAY PANEL – 100 CAPSULE BOTTLE CARTON – NDC 62541-401-10
- NDC 62541-401-10
- pancrelipase
PANCREAZE ®
Delayed-Release Capsules - Each capsule contains:
- Lipase 2,600 USP Units
Amylase 15,200 USP Units
Protease 8,800 USP Units - DOSE BY LIPASE UNITS
- Each delayed-release capsule
contains enteric coated pancrelipase
microtablets.
This product is of porcine origin. - Attention Pharmacist:
Dispense the accompanying
Medication Guide to each patient. - Rx only.
- 100
delayed-release
capsules
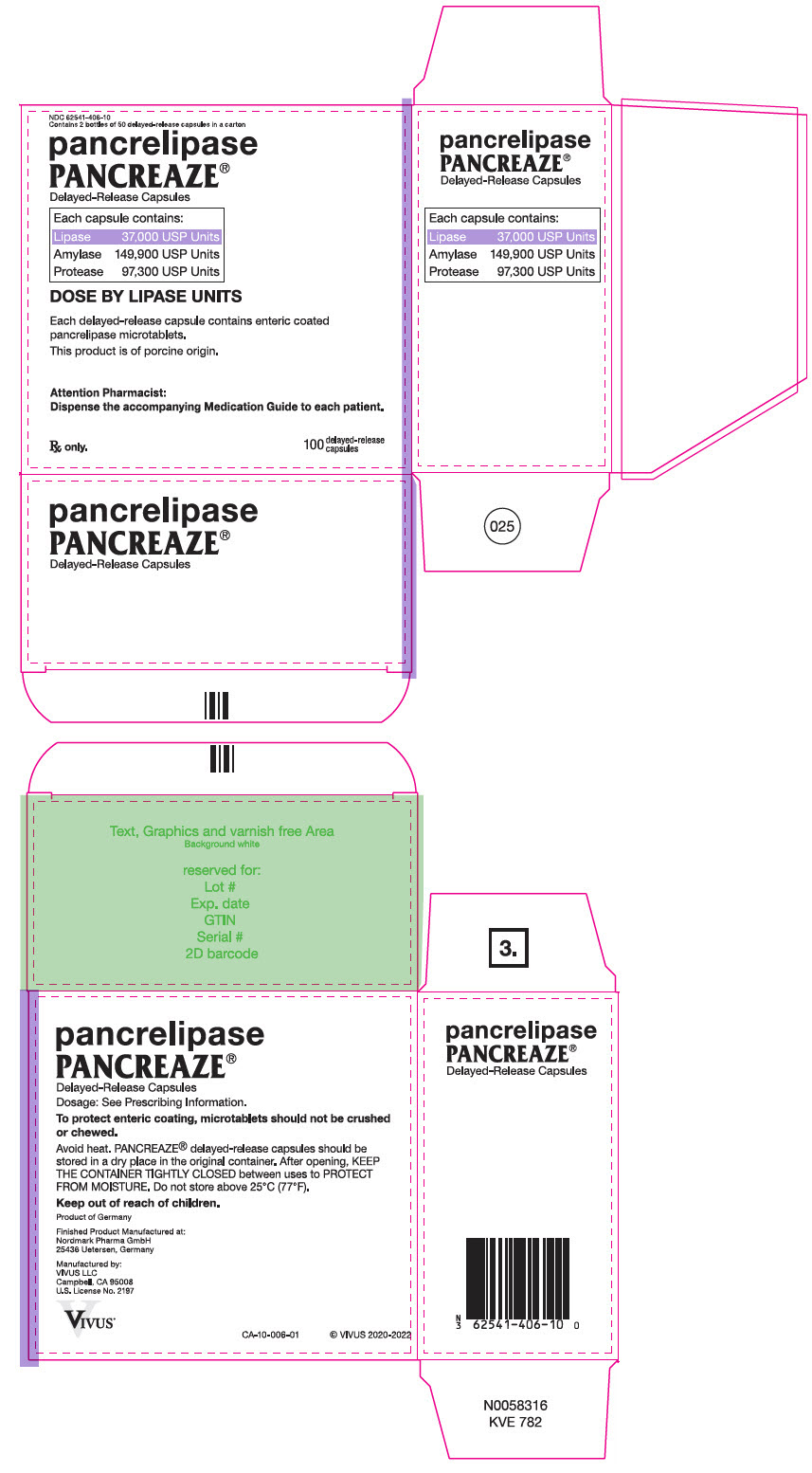
PRINCIPAL DISPLAY PANEL – 100 CAPSULE BOTTLE CARTON – NDC 62541-406-10
- NDC 62541-406-10
Contains 2 bottles of 50 delayed-release capsules in a carton - pancrelipase
PANCREAZE ®
Delayed-Release Capsules - Each capsule contains:
- Lipase 37,000 USP Units
Amylase 149,900 USP Units
Protease 97,300 USP Units - DOSE BY LIPASE UNITS
- Each delayed-release capsule contains enteric coated
pancrelipase microtablets.
This product is of porcine origin. - Attention Pharmacist:
Dispense the accompanying Medication Guide to each patient. - Rx only.
- 100
delayed-release
capsules
SRC: NLM .