Palynziq
Generic name: pegvaliase
Drug class: Miscellaneous metabolic agents
Medically reviewed by A Ras MD.
What is Palynziq?
Palynziq is a prescription medicine used to lower blood levels of phenylalanine in adults with PKU (phenylketonuria) who have uncontrolled blood phenylalanine levels above 600 micromol/L (10 mg/dL) on their current treatment.
It is not known if Palynziq is safe and effective in children.
Description
Pegvaliase-pqpz is a phenylalanine-metabolizing enzyme that is composed of recombinant phenylalanine ammonia lyase (rAvPAL) conjugated to N-hydroxysuccinimide (NHS)-methoxypolyethylene glycol (PEG). rAvPAL is manufactured in Escherichia coli bacteria transformed with a plasmid containing the phenylalanine ammonia lyase (PAL) gene derived from Anabaena variabilis. During the rAvPAL manufacturing process, fermentation is carried out in nutrient medium containing the antibiotic kanamycin. However, kanamycin is cleared in the manufacturing process and is not detectable in the final product. rAvPAL is a homotetrameric protein with a molecular weight of 62 kD per monomer. To produce pegvaliase-pqpz, an average of nine (9) 20 kD PEG molecules are covalently bound (or conjugated) to each monomer of rAvPAL. The total molecular weight of pegvaliase‑pqpz (rAvPAL‑PEG) is approximately 1000 kD.
Palynziq (pegvaliase‑pqpz) injection, intended for subcutaneous injection, is a clear to slightly opalescent, colorless to pale yellow, sterile, preservative-free solution and is formulated at pH 6.6 to 7.4.
Palynziq is provided in a single-dose prefilled syringe and is available in three dosage strengths: 2.5 mg/0.5 mL, 10 mg/0.5 mL, and 20 mg/mL. Palynziq contents for each dosage strength are summarized in Table 4.
|
Strength |
Total Contents per Prefilled Syringe |
|
Palynziq 2.5 mg/0.5 mL prefilled syringe |
2.5 mg pegvaliase-pqpz (expressed as the amount of rAvPAL conjugated to 7.25 mg of 20 kD PEG in 0.5 mL Water for Injection, USP) and contains the following inactive ingredients: sodium chloride (for tonicity adjustment), trans-cinnamic acid (0.07 mg), and tromethamine and tromethamine hydrochloride (for pH adjustment). |
|
Palynziq 10 mg/0.5 mL prefilled syringe |
10 mg pegvaliase-pqpz (expressed as the amount of rAvPAL conjugated to 29 mg of 20 kD PEG in 0.5 mL Water for Injection, USP) and contains the following inactive ingredients: sodium chloride (for tonicity adjustment), trans-cinnamic acid (0.07 mg), and tromethamine and tromethamine hydrochloride (for pH adjustment). |
|
Palynziq 20 mg/mL prefilled syringe |
20 mg pegvaliase-pqpz (expressed as the amount of rAvPAL conjugated to 58 mg of 20 kD PEG in 1 mL Water for Injection, USP) and contains the following inactive ingredients: sodium chloride (for tonicity adjustment), trans-cinnamic acid (0.15 mg), and tromethamine and tromethamine hydrochloride (for pH adjustment). |
Mechanism of Action
Pegvaliase-pqpz is a PEGylated phenylalanine ammonia lyase (PAL) enzyme that converts phenylalanine to ammonia and trans‑cinnamic acid. It substitutes for the deficient phenylalanine hydroxylase (PAH) enzyme activity in patients with PKU and reduces blood phenylalanine concentrations.
What is the most important information I should know about Palynziq?
Palynziq can cause a severe allergic reaction (anaphylaxis) that may be life-threatening and can happen any time during treatment with Palynziq. Severe allergic reactions are a serious but common side effect of Palynziq.
- You will receive your first injection of Palynziq in a healthcare setting where you will be closely watched for at least 1 hour after your injection for a severe allergic reaction.
- If you have a severe allergic reaction during treatment with Palynziq, you will need to receive an auto-injection of epinephrine immediately and get emergency medical help right away.
- Your healthcare provider will decide if you (or your caregiver) are able to give the Palynziq injections, recognize the signs and symptoms of a severe allergic reaction, give an injection of epinephrine and call for emergency medical help, if needed.
- Your healthcare provider may recommend that an adult observer (or your caregiver) be with you when you give your Palynziq injection, and for at least 1 hour after your injection to watch you for signs and symptoms of a severe allergic reaction and, if needed, give you an injection of epinephrine and call for emergency medical help.Stop injecting Palynziq and get emergency medical care right away if you have any of the following symptoms of a severe allergic reaction during treatment with Palynziq:
- fainting (passing out)
- dizziness or lightheadedness
- sudden confusion
- trouble breathing or wheezing
- chest discomfort or chest tightness
- fast heart rate
- swelling of your face, lips, eyes, or tongue
- throat tightness
- flushed skin
- skin rash, itching, or raised bumps on skin
- nausea, vomiting, or diarrhea
- losing control of urine or stools
- Your healthcare provider will prescribe an auto-injectable epinephrine for you and will teach you (or your caregiver) and your observer, if needed, when and how to use it if you have a severe allergic reaction. Keep the auto-injectable epinephrine with you at all times during treatment with Palynziq. Read the Patient Information that comes with the auto-injectable epinephrine that your healthcare provider prescribes for you for more information.
- If you have a severe allergic reaction, do not continue to take Palynziq until you talk with your healthcare provider. Tell your healthcare provider that you had a severe allergic reaction. Your healthcare provider will tell you if you can continue treatment with Palynziq.
- Your healthcare provider may prescribe other medicines to take before your Palynziq injection that may help reduce the symptoms of an allergic reaction.
- If your healthcare provider decides that you can continue treatment with Palynziq after a severe allergic reaction, you will receive your next injection of Palynziq in a healthcare setting where you will be closely watched for at least 1 hour after your injection for a severe allergic reaction.
- Your healthcare provider will give you a Palynziq Patient Wallet Card that describes symptoms that you (or your caregiver), or your observer, should know that require you to get emergency medical care right away. Carry this card with you at all times during treatment with Palynziq. It is important to show your Palynziq Patient Wallet Card to any other healthcare provider who treats you.
Palynziq is only available through a restricted program called the Palynziq Risk Evaluation and Mitigation Strategy (REMS) Program. Before you can receive Palynziq, you must:
- enroll in this program.
- receive education about the risk of a severe allergic reaction (anaphylaxis) by a healthcare provider certified in the Palynziq REMS to be sure that you understand the risks and benefits of treatment with Palynziq.
- fill the prescription your healthcare provider gives you for the auto-injectable epinephrine and carry it with you at all times during treatment with Palynziq.
- carry the Palynziq Patient Wallet Card with you at all times.
Talk to your healthcare provider for more information about the Palynziq REMS and how to enroll.
What should I tell my healthcare provider before using Palynziq?
Before injecting Palynziq, tell your healthcare provider about all of your medical conditions, including if you:
- cannot or are not willing to use auto-injectable epinephrine to treat a severe allergic reaction.
- are pregnant or plan to become pregnant. Palynziq may harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you might be pregnant while taking Palynziq.
- If your phenylalanine levels are too high or too low during pregnancy, this may also affect your unborn baby. You and your healthcare provider can decide the best way for you to manage your blood phenylalanine levels and discuss the risks and benefits of taking Palynziq during pregnancy to you and your unborn baby. It is very important to keep your phenylalanine levels at the levels your healthcare provider recommends during pregnancy.
- Pregnancy Surveillance Program. There is a pregnancy surveillance program for females who take Palynziq during pregnancy, or who become pregnant while receiving Palynziq or within 1 month after their last dose of Palynziq. The purpose of this program is to collect information about the health of you and your baby while taking Palynziq. Talk to your healthcare provider about how you can take part in this program or call BioMarin at 1-866-906-6100.
- are breastfeeding or plan to breastfeed. It is not known if Palynziq passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Palynziq.
Tell your healthcare provider about all the medicines you take, including prescription or over-the-counter medicines, vitamins, or herbal supplements.
How should I use Palynziq?
- Your healthcare provider will give you the Palynziq injection until they decide that you (or your caregiver) can give it. See “What is the most important information I should know about Palynziq?”
- Your healthcare provider may prescribe medicine for you to take before your Palynziq injection to help reduce the symptoms of an allergic reaction.
- If your healthcare provider decides that you (or your caregiver) can give your Palynziq injections, you (or your caregiver) will be shown how to prepare and give your Palynziq injections. See the “Instructions for Use” the come with Palynziq for detailed instructions on how to prepare and give an injection of Palynziq.
- Use Palynziq exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much Palynziq to inject and when to inject it.
- Palynziq comes in prefilled syringes with 3 different strengths (2.5 mg, 10 mg, or 20 mg). You may need more than 1 Palynziq prefilled syringe for your prescribed dose.
- Monitor the amount of protein and phenylalanine that you eat or drink. Your healthcare provider may change the amount of protein and phenylalanine you should have in your diet during treatment with Palynziq, depending on the levels of phenylalanine in your blood. Follow your healthcare provider’s instructions about the amount of protein and phenylalanine you should have in your diet.
- If you miss a dose, take your next dose at the regular time. Do not take two doses of Palynziq to make up for a missed dose.
What are the possible side effects of Palynziq?
Palynziq may cause serious side effects, including:
- See “What is the most important information I should know about Palynziq?”
- Other allergic reactions to Palynziq can happen during treatment with Palynziq. Contact your healthcare provider right away if you have any of the following symptoms of an allergic reaction including: rash, itching, or swelling of the face, lips, eyes, or tongue. Your healthcare provider may change your dose of Palynziq, stop your treatment with Palynziq for a period of time, or prescribe medicine for you to take before your Palynziq injection to help reduce the symptoms of an allergic reaction.
The most common side effects of Palynziq include:
- injection site reactions, such as redness, itching, pain, bruising, rash, swelling, tenderness
- joint pain
- headache
- skin reactions that spread on your skin and last at least 14 days, such as itching, rash, redness
- itching
- nausea
- stomach pain
- mouth and throat pain
- vomiting
- cough
- diarrhea
- feel very tired
- low levels of phenylalanine in your blood
These are not all the possible side effects of Palynziq. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Palynziq
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Palynziq for a condition for which it was not prescribed. Do not give Palynziq to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Palynziq that is written for health professionals.
How should I store Palynziq?
- Store Palynziq in the refrigerator at 36°F to 46°F (2°C to 8°C).
- If needed, you may store Palynziq at room temperature between 68°F to 77°F (20°C to 25°C) for up to 30 days.
- Write the date that you remove Palynziq from the refrigerator on the carton.
- If stored at room temperature, do not put Palynziq back in the refrigerator.
- Keep Palynziq in the original carton to protect from light.
- Do not freeze or shake Palynziq.
- Throw away Palynziq if it has been kept at room temperature for 30 days and has not been used, or after the expiration date on the carton, whichever comes first.
Keep Palynziq and all medicines out of the reach of children.
What are the ingredients in Palynziq?
Active ingredients: pegvaliase-pqpz
Inactive ingredients: sodium chloride, trans-cinnamic acid, tromethamine, and tromethamine hydrochloride
Label
PALYNZIQ – 2.5 MG/0.5 ML
- NDC 68135-058-90
- Rx only
- PalynziqTM
- (pegvaliase-pqpz)
- Injection
- 2.5 mg/0.5 mL
- For Subcutaneous Use Only
- Contains one x 2.5 mg/0.5 mL single-dose prefilled syringe.
- Discard after use.
- ATTENTION: Dispense the enclosed Medication Guide to each patient.



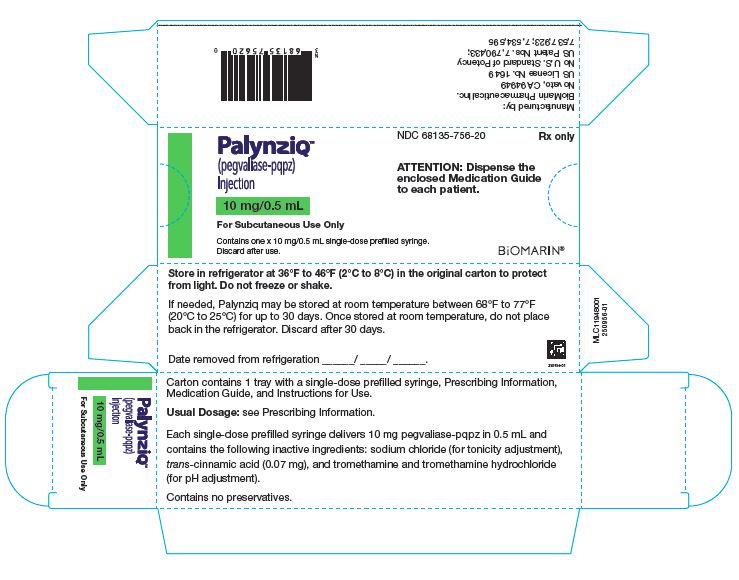
PALYNZIQ – 10 MG/0.5ML
- NDC 68135-756-20
- Rx only
- PalynziqTM
- (pegvaliase-pqpz)
- Injection
- 10 mg/0.5 mL
- For Subcutaneous Use Only
- Contains one x 10 mg/0.5 mL single-dose prefilled syringe.
- Discard after use.
- ATTENTION: Dispense the enclosed Medication Guide to each patient.


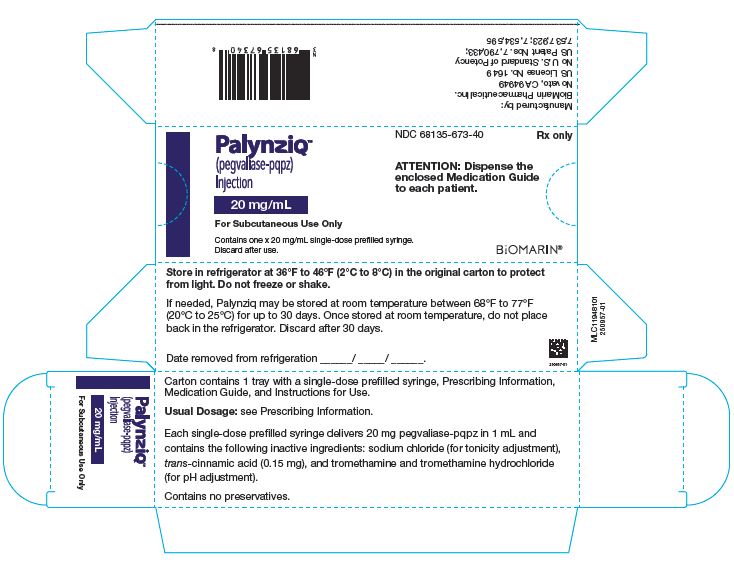
PALYNZIQ (SINGLE PACK) – 20 MG/ML
- NDC 68135-673-40
- Rx only
- PalynziqTM
- (pegvaliase-pqpz)
- Injection
- 20 mg/mL
- For Subcutaneous Use Only
- Contains one x 20 mg/mL single-dose prefilled syringe.
- Discard after use.
- ATTENTION: Dispense the enclosed Medication Guide to each patient.


SRC: NLM .
