Opzelura
Generic name: ruxolitinib
Dosage form: cream for topical use
Drug class: Topical antineoplastics
Medically reviewed by A Ras MD.
What is Opzelura?
Opzelura is a prescription medicine used on the skin (topical) for short-term and non-continuous treatment of mild to moderate eczema (atopic dermatitis) in non-immunocompromised people 12 and older whose disease is not well controlled with topical prescription therapies or when those therapies are not recommended
The use of Opzelura along with therapeutic biologics for atopic dermatitis, other JAK inhibitors, or strong immunosuppressants such as azathioprine or cyclosporine is not recommended.
It is not known if Opzelura is safe or effective in children less than 12 years of age.
Description
Ruxolitinib phosphate is a Janus kinase inhibitor with the chemical name (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate and a molecular weight of 404.36. Ruxolitinib phosphate has the following structural formula:
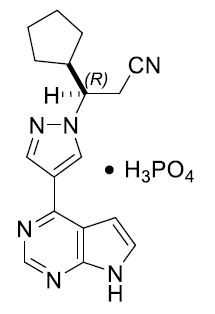
Ruxolitinib phosphate is a white to off-white to light pink powder.
OPZELURA (ruxolitinib) cream is a white to off-white oil-in-water, solubilized emulsion cream for topical use.
Each gram of OPZELURA contains 15 mg of ruxolitinib (equivalent to 19.8 mg of ruxolitinib phosphate) in a cream containing cetyl alcohol, dimethicone 350, edetate disodium, glyceryl stearate SE, light mineral oil, medium chain triglycerides, methylparaben, phenoxyethanol, polyethylene glycol 200, polysorbate 20, propylene glycol, propylparaben, stearyl alcohol, purified water, white petrolatum, and xanthan gum.
Mechanism of Action
Ruxolitinib, a Janus kinase (JAK) inhibitor, inhibits JAK1 and JAK2 which mediate the signaling of a number of cytokines and growth factors that are important for hematopoiesis and immune function. JAK signaling involves recruitment of STATs (signal transducers and activators of transcription) to cytokine receptors, activation and subsequent localization of STATs to the nucleus leading to modulation of gene expression. The relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.
What is the most important information I should know about Opzelura?
Important: Opzelura cream is for use on the skin only. Do not use Opzelura cream, in your eyes, mouth or vagina.
Opzelura may cause serious side effects, including:
- Serious Infections. Opzelura contains ruxolitinib. Ruxolitinib belongs to a class of medicines called Janus kinase (JAK) inhibitors. JAK inhibitors are medicines that affect your immune system. JAK inhibitors can lower the ability of your immune system to fight infections. Some people have had serious infections while taking JAK inhibitors by mouth, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have been hospitalized or died from these infections. Some people have had serious infections of their lungs while taking Opzelura.
- Your healthcare provider should watch you closely for signs and symptoms of TB during treatment with Opzelura.
Opzelura should not be used in people with an active, serious infection, including localized infections. You should not start using Opzelura if you have any kind of infection unless your healthcare provider tells you it is okay. You may be at a higher risk of developing shingles (herpes zoster) while using Opzelura.
Before starting Opzelura, tell your healthcare provider if you:
- are being treated for an infection
- have had an infection that does not go away or that keeps coming back
- have diabetes, chronic lung disease, HIV, or a weak immune system
- have TB or have been in close contact with someone with TB
- have had shingles (herpes zoster)
- have had hepatitis B or C
- live in an area, or have lived in an area, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance for getting certain kinds of fungal infections. These infections may happen or become more severe if you use Opzelura. Ask your healthcare provider if you do not know if you have lived in an area where these infections are common.
- think you have an infection or have symptoms of an infection such as:
- fever, sweating, or chills
- muscle aches
- cough or shortness of breath
- blood in your phlegm
- weight loss
- warm, red, or painful skin or sores on your body
- diarrhea or stomach pain
- burning when you urinate or urinating more often than usual
- feeling very tired
After starting Opzelura, call your healthcare provider right away if you have any symptoms of an infection.
Opzelura can make you more likely to get infections or make worse any infections that you have.
- Increased risk of death from all causes, including sudden cardiac death, has happened in people taking JAK inhibitors by mouth.
- Cancer and immune system problems. Opzelura may increase your risk of certain cancers by changing the way your immune system works. Some people have had lymphoma and other cancers while taking JAK inhibitors by mouth, especially if they are a current or past smoker. Some people have had skin cancers while taking Opzelura. Your healthcare provider will regularly check your skin during your treatment with Opzelura. Tell your healthcare provider if you have ever had any type of cancer.
- Increased risk of major cardiovascular events such as heart attack, stroke or death has happened in people with cardiovascular risk factors and who are current or past smokers while using JAK inhibitors to treat inflammatory conditions.Get emergency help right away if you have any symptoms of a heart attack or stroke while using Opzelura, including:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
- weakness in one part or on one side of your body
- slurred speech
- Blood clots. Blood clots in the veins of your legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) can happen in some people taking Opzelura. This may be life-threatening.
- Tell your healthcare provider if you have had blood clots in the veins of your legs or lungs in the past.
- Tell your healthcare provider right away if you have any signs and symptoms of blood clots during treatment with Opzelura, including:
- swelling, pain or tenderness in one or both legs
- sudden, unexplained chest or upper back pain
- shortness of breath or difficulty breathing
See “What are the possible side effects of Opzelura?” for more information about side effects.
What should I tell my healthcare provider before using Opzelura?
Before using Opzelura, tell your healthcare provider about all of your medical conditions, including if you:
- have an infection
- have or had tuberculosis (TB), or have been in close contact with someone who has TB
- have had shingles (herpes zoster)
- have or had hepatitis B or C
- have had skin cancer in the past
- are a current or past smoker
- have or have had low white or red blood cell counts
- have high levels of fat in your blood (high cholesterol or triglycerides)
- are pregnant or plan to become pregnant. It is not known if Opzelura will harm your unborn baby.
- Pregnancy Exposure Registry. There is a pregnancy exposure registry for individuals who use Opzelura during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. If you become exposed to Opzelura during pregnancy, you and your healthcare provider should report exposure to Incyte Corporation at 1-855-463-3463.
- are breastfeeding or plan to breastfeed. It is not known if Opzelura passes into your breast milk. Do not breastfeed during treatment with Opzelura and for about 4 weeks after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Opzelura?
- Opzelura is for use on the skin only. Do not use Opzelura in your eyes, mouth, or vagina.
- Use Opzelura exactly as your healthcare provider tells you.
- Apply a thin layer of Opzelura 2 times a day to affected areas. Do not use more than one 60-gram tube each week. Ask your healthcare provider if you have questions about applying Opzelura.
- Wash your hands after applying Opzelura, unless hands are being treated. If someone else applies Opzelura, they should wash their hands after applying Opzelura.
What are the possible side effects of Opzelura?
Opzelura may cause serious side effects, including:
- See “What is the most important information I should know about Opzelura?”
- Low blood cell counts. Opzelura may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia), and low white blood cell counts (neutropenia). If needed, your healthcare provider will do a blood test to check your blood cell counts during your treatment with Opzelura and may stop your treatment if signs or symptoms of low blood cell counts happen. Tell your healthcare provider right away if you develop or have worsening of any of these symptoms:
- unusual bleeding
- bruising
- tiredness
- shortness of breath
- fever
- Cholesterol increases. Cholesterol increase has happened in people when ruxolitinib is taken by mouth. Tell your healthcare provider if you have high levels of fat in your blood (high cholesterol or triglycerides).
The most common side effects of Opzelura include:
- pain or swelling in your nose or throat (nasopharyngitis)
- diarrhea
- bronchitis
- ear infection
- increase in a type of white blood cell (eosinophil) counts
- hives
- inflamed hair pores (folliculitis)
- swelling of the tonsils (tonsillitis)
- runny nose (rhinorrhea)
These are not all of the possible side effects of Opzelura.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800-FDA-1088.
You may also report side effects to Incyte Corporation at 1-855-463-3463.
General information about the safe and effective use of Opzelura
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Opzelura for a condition for which it is not prescribed. Do not give Opzelura to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Opzelura that is written for healthcare professionals.
How should I store Opzelura?
- Store Opzelura at room temperature from 68°F to 77°F (20°C to 25°C).
Keep Opzelura and all medicines out of the reach of children.
What are the ingredients in Opzelura?
Active ingredient: ruxolitinib phosphate
Inactive ingredients: cetyl alcohol, dimethicone 350, edetate disodium, glyceryl stearate SE, light mineral oil, medium chain triglycerides, methylparaben, phenoxyethanol, polyethylene glycol 200, polysorbate 20, propylene glycol, propylparaben, stearyl alcohol, purified water, white petrolatum, and xanthan gum.
Label
100 G CARTON LABEL
- Opzelura™
(ruxolitinib) cream 1.5% - For Topical Use Only.
- NDC 50881-007-07
- 100 g
Rx only
60 G CARTON LABEL
- Opzelura™
(ruxolitinib) cream 1.5% - For Topical Use Only.
- NDC 50881-007-05
- 60 g
Rx only

5 G CARTON LABEL – SAMPLE
- Opzelura™
(ruxolitinib) cream 1.5% - Sample – Topical Use Only.
- NDC 50881-007-04
- 5 g
Rx only
SRC: NLM .
