Nyvepria
Generic name: pegfilgrastim
Brand names: Fulphila, Neulasta, Neulasta Onpro Kit, Nyvepria, Udenyca, Ziextenzo
Drug class: Colony stimulating factors
Medically reviewed by A Ras MD.
What is Nyvepria?
Nyvepria is a man-made form of granulocyte colony-stimulating factor (G-CSF). G-CSF is a substance produced by the body. It stimulates the growth of neutrophils, a type of white blood cell important in the body’s fight against infection.
Description
Pegfilgrastim-apgf is a covalent conjugate of recombinant methionyl human G-CSF and monomethoxypolyethylene glycol. Recombinant methionyl human G-CSF is a water-soluble 175 amino acid protein with a molecular weight of approximately 19 kilodaltons (kD). Recombinant methionyl human G-CSF is obtained from the bacterial fermentation of a strain of E. coli transformed with a genetically engineered plasmid containing the human G-CSF gene. To produce pegfilgrastim-apgf, a 20 kD monomethoxypolyethylene glycol molecule is covalently bound to the N-terminal methionyl residue of recombinant methionyl human G-CSF. The average molecular weight of pegfilgrastim-apgf is approximately 39 kD.
NYVEPRIA for manual subcutaneous injection is supplied in 0.6 mL prefilled syringes. The prefilled syringe does not bear graduation marks and is designed to deliver the entire contents of the syringe (6 mg/0.6 mL).
The delivered 0.6 mL dose from the prefilled syringe for manual subcutaneous injection contains 6 mg pegfilgrastim-apgf (based on protein weight) in a sterile, clear, colorless, preservative-free solution (pH 4.0) containing acetate (0.35 mg), polysorbate 20 (0.02 mg), sodium (0.01 mg), and sorbitol (30 mg) in Water for Injection, USP.
Mechanism of Action
Pegfilgrastim products are colony-stimulating factors that act on hematopoietic cells by binding to specific cell surface receptors, thereby stimulating proliferation, differentiation, commitment, and end cell functional activation.
Who should not take Nyvepria?
Do not take Nyvepria if you have had a serious allergic reaction to pegfilgrastim products or filgrastim products
What should I tell my healthcare provider before taking Nyvepria?
Before you receive Nyvepria, tell your healthcare provider about all of your medical conditions, including if you:
- have a sickle cell disorder.
- have kidney problems.
- are pregnant or plan to become pregnant. It is not known if Nyvepria will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Nyvepria passes into your breast milk.
Tell your healthcare provider about all of the medicines you take, including prescription and over-thecounter medicines, vitamins, and herbal supplements
How should I use Nyvepria?
- Nyvepria is given as an injection under your skin (subcutaneous injection) by a healthcare provider. If your healthcare provider decides that the subcutaneous injections can be given at home by you or your caregiver, follow the detailed instructions for use that comes with your Nyvepria for information on how to prepare and inject a dose of Nyvepria.
- You and your caregiver will be shown how to prepare and inject Nyvepria before you use it.
- You should not inject a dose of Nyvepria to children weighing less than 45 kg from a Nyvepria prefilled syringe. A dose less than 0.6 mL (6 mg) cannot be accurately measured using the Nyvepria prefilled syringe.
- If you are receiving Nyvepria because you are also receiving chemotherapy, the last dose of Nyvepria should be injected at least 14 days before and 24 hours after your dose of chemotherapy.
- If you miss a dose of Nyvepria, talk to your healthcare provider about when you should give your next dose.
What are the possible side effects of Nyvepria?
Nyvepria may cause serious side effects, including:
- Spleen rupture. Your spleen may become enlarged and can rupture. A ruptured spleen can cause death. Call your healthcare provider right away if you have pain in the left upper stomach area or your left shoulder.
- A serious lung problem called Acute Respiratory Distress Syndrome (ARDS). Call your healthcare provider or get emergency help right away if you have shortness of breath with or without a fever, trouble breathing, or a fast rate of breathing.
- Serious allergic reactions. Nyvepria can cause serious allergic reactions. These reactions can cause a rash over your whole body, shortness of breath, wheezing, dizziness, swelling around your mouth or eyes, fast heart rate, and sweating. If you have any of these symptoms, stop using Nyvepria and call your healthcare provider or get emergency medical help right away.
- Sickle cell crises. You may have a serious sickle cell crisis, which could lead to death, if you have a sickle cell disorder and receive Nyvepria. Call your healthcare provider right away if you have symptoms of sickle cell crisis such as pain or difficulty breathing.
- Kidney injury (glomerulonephritis). Nyvepria can cause kidney injury. Call your healthcare provider right away if you develop any of the following symptoms:
- swelling of your face or ankles
- blood in your urine or dark colored urine
- you urinate less than usual
- Increased white blood cell count (leukocytosis). Your healthcare provider will check your blood during treatment with Nyvepria.
- Capillary Leak Syndrome. Nyvepria can cause fluid to leak from blood vessels into your body’s tissues. This condition is called “Capillary Leak Syndrome” (CLS). CLS can quickly cause you to have symptoms that may become life-threatening. Get emergency medical help right away if you develop any of the following symptoms:
- swelling or puffiness and are urinating less than usual
- trouble breathing
- swelling of your stomach area (abdomen) and feeling of fullness
- dizziness or feeling faint
- a general feeling of tiredness
- Inflammation of the aorta (aortitis). Inflammation of the aorta (the large blood vessel which transports blood from the heart to the body) has been reported in patients who received pegfilgrastim products. Symptoms may include fever, abdominal pain, feeling tired, and back pain. Call your healthcare provider if you experience these symptoms.
The most common side effects of Nyvepria are pain in the bones, arms, and legs. These are not all the possible side effects of Nyvepria. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Nyvepria
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Nyvepria for a condition for which it was not prescribed. Do not give Nyvepria to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Nyvepria that is written for health professionals.
How should I store Nyvepria?
- Store Nyvepria in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze. If Nyvepria is accidentally frozen, allow the prefilled syringe to thaw in the refrigerator before injecting.
- Do not use a Nyvepria prefilled syringe that has been frozen more than 1 time. Use a new Nyvepria prefilled syringe.
- Keep the prefilled syringe in the original carton to protect from light or physical damage.
- Do not shake the prefilled syringe.
- Take Nyvepria out of the refrigerator 30 minutes before use and allow it to reach room temperature before preparing an injection.
- Throw away (dispose of) any Nyvepria that has been left at room temperature, 68°F to 77°F (20°C to 25°C), for more than 15 days.
Keep the Nyvepria prefilled syringe out of the reach of children.
What are the ingredients in Nyvepria?
Active ingredient: pegfilgrastim-apgf.
Inactive ingredients: acetate, polysorbate 20, sodium, and sorbitol in water for injection.
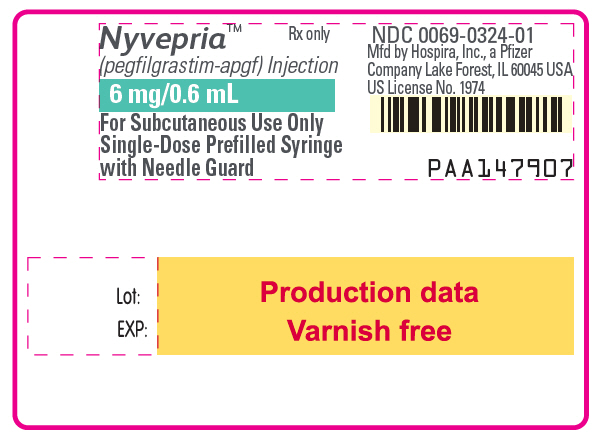
Label
PRINCIPAL DISPLAY PANEL – 6 MG/0.6 ML SYRINGE LABEL
- Nyvepria™
(pegfilgrastim-apgf) Injection - Rx only
- 6 mg/0.6 mL
- For Subcutaneous Use Only
Single-Dose Prefilled Syringe
with Needle Guard - NDC 0069-0324-01
Mfd by Hospira, Inc., a Pfizer
Company Lake Forest, IL 60045 USA
US License No. 1974 - PAA147907
PRINCIPAL DISPLAY PANEL – 6 MG/0.6 ML SYRINGE CARTON – PAA147908
- Nyvepria™
(pegfilgrastim-apgf) Injection - 6 mg/0.6 mL
- For Subcutaneous Use Only
Sterile Solution – No Preservative - 1 x 6 mg/0.6 mL
Single-Dose Prefilled Syringe
with Needle Guard - Pegylated Recombinant Methionyl Human
Granulocyte Colony-Stimulating Factor
(PEG-r-metHuG-CSF) derived from E coli - NDC 0069-0324-01
- Rx Only

SRC: NLM .
