Myfortic
Generic name: mycophenolic acid
Drug class: Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Myfortic?
Myfortic is a prescription medicine given to prevent rejection (antirejection medicine) in people who have received a kidney transplant. Rejection is when the body’s immune system senses the new organ as “foreign” and attacks it.
Myfortic is used with other medicines containing cyclosporine (Sandimmune, Gengraf, and Neoral) and corticosteroids.
Myfortic can be used to prevent rejection in children who are 5 years or older and are stable after having a kidney transplant. It is not known if Myfortic is safe and works in children younger than 5 years. It is not known how Myfortic works in children who have just received a new kidney transplant.
Description
Myfortic® (mycophenolic acid) delayed-release tablets are an enteric formulation of mycophenolate sodium that delivers the active moiety mycophenolic acid (MPA). Myfortic is an immunosuppressive agent. As the sodium salt, MPA is chemically designated as (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoic acid sodium salt.
Its empirical formula is C17H19O6Na. The molecular weight is 342.32 g/mol and the structural formula is:
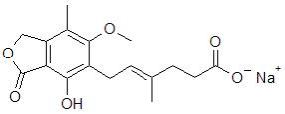
Myfortic, as the sodium salt, is a white to off-white, crystalline powder and is highly soluble in aqueous media at physiological pH and practically insoluble in 0.1N hydrochloric acid.
Myfortic is available for oral use as delayed-release tablets containing either 180 mg or 360 mg of mycophenolic acid. Inactive ingredients include colloidal silicon dioxide, crospovidone, lactose anhydrous, magnesium stearate, povidone (K-30), and starch. The enteric coating of the tablet consists of hypromellose phthalate, titanium dioxide, iron oxide yellow, and indigotine (180 mg) or iron oxide red (360 mg).
Mechanism of Action
Mycophenolic acid (MPA), an immunosuppressant, is an uncompetitive and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), and therefore inhibits the de novo pathway of guanosine nucleotide synthesis without incorporation to DNA. T- and B-lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, whereas other cell types can utilize salvage pathways. MPA has cytostatic effects on lymphocytes.
Mycophenolate sodium has been shown to prevent the occurrence of acute rejection in rat models of kidney and heart allotransplantation. Mycophenolate sodium also decreases antibody production in mice.
What is the most important information I should know about Myfortic?
Myfortic can cause serious side effect, including:
- Increased risk of loss of pregnancy (miscarriage) and higher risk of birth defects. Females who take Myfortic during pregnancy, have a higher risk of miscarriage during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects.
- If you are a female who can become pregnant:
- your doctor must talk with you about acceptable birth control methods (contraceptive counseling) while taking Myfortic.
- you should have a pregnancy test immediately before starting Myfortic and another pregnancy test 8 to 10 days later. Pregnancy tests should be repeated during routine follow-up visits with your doctor. Talk to your doctor about the results of all of your pregnancy tests.
- you must use acceptable birth control during your entire Myfortic therapy and for 6 weeks after stopping Myfortic, unless at any time you choose to avoid sexual intercourse (abstinence) with a man completely. Myfortic decreases blood levels of the hormones in birth control pills that you take by mouth. Birth control pills may not work as well while you take Myfortic and you could become pregnant. If you decide to take birth control pills while using Myfortic, you must also use another form of birth control. Talk to your doctor about other birth control methods that can be used while taking Myfortic.
- If you are a sexually active male whose female partner can become pregnant while you are taking Myfortic, use effective contraception during treatment and for at least 90 days after stopping Myfortic.
- If you plan to become pregnant, talk with your doctor. Your doctor will decide if other medicines to prevent rejection may be right for you.
- If you become pregnant while taking Myfortic, do not stop taking Myfortic. Call your doctor right away. You and your doctor may decide that other medicines to prevent rejection may be right for you. You and your doctor should report your pregnancy to Mycophenolate Pregnancy Registry (1-800-617-8191). The purpose of this registry is to gather information about the health of your baby.
- If you are a female who can become pregnant:
- Increased risk of getting serious infections. Myfortic weakens the body’s immune system and affects your ability to fight infections. Serious infections can happen with Myfortic and can lead to death. These serious infections can include:
- Viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with Myfortic include:
- Shingles, other herpes infections, and cytomegalovirus (CMV). CMV can cause serious tissue and blood infections.
- BK virus. BK virus can affect how your kidney works and cause your transplanted kidney to fail.
- Hepatitis B and C viruses. Hepatitis viruses can affect how your liver works. Talk to your doctor about how hepatitis viruses may affect you.
- A brain infection called Progressive Multifocal Leukoencephalopathy (PML). In some patients Myfortic may cause an infection of the brain that may cause death. You are at risk for this brain infection because you have a weakened immune system. You should tell your healthcare provider right away if you have any of the following symptoms:
- Weakness on one side of the body
- You do not care about things that you usually care about (apathy)
- You are confused or have problems thinking
- You cannot control your muscles
- Fungal infections. Yeast and other types of fungal infections can happen with Myfortic and cause serious tissue and blood infections. See “What are the possible side effects of Myfortic?”Call your doctor right away if you have any of these signs and symptoms of infection:
- Temperature of 100.5°F or greater
- Cold symptoms, such as a runny nose or sore throat
- Flu symptoms, such as an upset stomach, stomach pain, vomiting, or diarrhea
- Earache or headache
- Pain during urination or you need to urinate often
- White patches in the mouth or throat
- Unexpected bruising or bleeding
- Cuts, scrapes, or incisions that are red, warm, and oozing pus
- Viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with Myfortic include:
- Increased risk of getting certain cancers. People who take Myfortic have a higher risk of getting lymphoma, and other cancers, especially skin cancer. Tell your doctor if you have:
- unexplained fever, tiredness that does not go away, weight loss, or lymph node swelling
- a brown or black skin lesion with uneven borders, or one part of the lesion does not look like other parts
- a change in the size or color of a mole
- a new skin lesion or bump
- any other changes to your health
See the section “What are the possible side effects of Myfortic?” for other serious side effects.
Who should not take Myfortic?
Do not take Myfortic if you are allergic to mycophenolic acid, mycophenolate sodium, mycophenolate mofetil, or any of the ingredients in Myfortic. See the end of this Medication Guide for a complete list of ingredients in Myfortic.
What should I tell my healthcare provider before taking Myfortic?
Tell your healthcare provider about all of your medical conditions, including if you:
- have any digestive problems, such as ulcers
- plan to receive any vaccines. You should not receive live vaccines while you take Myfortic. Some vaccines may not work as well during treatment with Myfortic.
- have Lesch-Nyhan or Kelley-Seegmiller syndrome or another rare inherited deficiency of hypoxanthine-guanine phosphoribosyl-transferase (HGPRT). You should not take Myfortic if you have one of these disorders.
- are pregnant or planning to become pregnant. See “What is the most important information I should know about Myfortic?”
- are breastfeeding or plan to breastfeed. It is not known if Myfortic passes into breast milk. You and your doctor will decide if you will breastfeed while taking Myfortic.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Some medicines may affect the way Myfortic works and Myfortic may affect how some medicines work. Especially tell your doctor if you take:
- birth control pills (oral contraceptives). See “What is the most important information I should know about Myfortic?”
- antacids that contain aluminum or magnesium. Myfortic and antacids should not be taken at the same time.
- acyclovir (Zovirax), Ganciclovir (Cytovene IV, Valcyte)
- azathioprine (Azasan, Imuran)
- cholestyramine (Questran Light, Questran, Locholest Light, Prevalite)
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine. Do not take any new medicine without talking to your doctor.
How should I take Myfortic?
- Take Myfortic exactly as prescribed. Your healthcare provider will tell you how much Myfortic to take.
- Do not stop taking or change your dose of Myfortic without talking to your healthcare provider.
- Take Myfortic on an empty stomach, either 1 hour before or 2 hours after a meal.
- Swallow Myfortic whole. Do not crush, chew, or cut Myfortic. The Myfortic tablets have a coating so that the medicine will pass through your stomach and dissolve in your intestine.
- If you forget to take Myfortic, take it as soon as you remember and then take your next dose at its regular time. If it is almost time for your next dose, skip the missed dose. Do not take two doses at the same time. Call your doctor or pharmacist if you are not sure what to do.
- If you take more than the prescribed dose of Myfortic, call your doctor right away.
- Do not change (substitute) between using Myfortic delayed-release tablets and mycophenolate mofetil tablets, capsules, or oral suspension for one another unless your healthcare provider tells you to. These medicines are absorbed differently. This may affect the amount of medicine in your blood.
- Be sure to keep all appointments at your transplant clinic. During these visits, your doctor may perform regular blood tests.
What should I avoid while taking Myfortic?
- Avoid pregnancy. See “What is the most important information I should know about Myfortic?”
- Limit the amount of time you spend in sunlight. Avoid using tanning beds and sunlamps. People who take Myfortic have a higher risk of getting skin cancer. See “What is the most important information I should know about Myfortic?” Wear protective clothing when you are in the sun and use a sunscreen with a high sun protection factor (SPF 30 and above). This is especially important if your skin is fair (light colored) or you have a family history of skin cancer.
- You should not donate blood while taking Myfortic and for at least 6 weeks after stopping Myfortic.
- You should not donate sperm while taking Myfortic and for 90 days after stopping Myfortic.
- Elderly patients 65 years of age or older may have more side effects with Myfortic because of a weaker immune system.
What are the possible side effects of Myfortic?
Myfortic can cause serious side effects.
See “What is the most important information I should know about Myfortic?”
Stomach and intestinal bleeding can happen in people who take Myfortic. Bleeding can be severe and you may have to be hospitalized for treatment.
The most common side effects of taking Myfortic include:
In people with a new transplant:
- low blood cell counts
- red blood cells
- white blood cells
- platelets
- constipation
- nausea
- diarrhea
- vomiting
- urinary tract infections
- stomach upset
In people who take Myfortic for a long time (long-term) after transplant:
- low blood cell counts
- red blood cells
- white blood cells
- nausea
- diarrhea
- sore throat
Your healthcare provider will do blood tests before you start taking Myfortic and during treatment with Myfortic to check your blood cell counts. Tell your healthcare provider right away if you have any signs of infection (see “What is the most important information I should know about Myfortic?”), or any unexpected bruising or bleeding. Also, tell your healthcare provider if you have unusual tiredness, dizziness, or fainting.
These are not all the possible side effects of Myfortic. Your healthcare provider may be able to help you manage these side effects.
Call your doctor for medical advice about side effects. You may report side effects to
- FDA MedWatch at 1-800-FDA-1088 or
- Novartis Drug Safety at 888-NOW-NOVA (1-888-669-6682).
General information about the safe and effective use of Myfortic
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Myfortic for a condition for which it was not prescribed. Do not give Myfortic to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about Myfortic. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Myfortic that is written for healthcare professionals. You can also call 1-888-669-6682 or visit the Myfortic website at www.myfortic.com.
How should I store Myfortic?
- Store Myfortic tablets at room temperature, 59° to 86°F (15° to 30°C). Myfortic does not need to be refrigerated.
- Keep the container tightly closed. Store Myfortic in a dry place.
- Keep Myfortic and all medicines out of the reach of children.
What are the ingredients in Myfortic?
Active ingredient: mycophenolic acid (as mycophenolate sodium)
Inactive ingredients: colloidal silicon dioxide, crospovidone, lactose anhydrous, magnesium stearate, povidone (K-30), and starch. The enteric coating of the tablet consists of hypromellose phthalate, titanium dioxide, iron oxide yellow, and indigotine (for the 180-mg tablet) or iron oxide red (for the 360-mg tablet)
Label
PRINCIPAL DISPLAY PANEL
- NOVARTIS
- NDC 0078-0385-66
- Myfortic®
- (mycophenolic acid*)
- delayed-release tablets
- *as mycophenolate sodium
- Dispense the accompanying Medication Guide to each patient.
- 120 Tablets
- 180 mg
- Rx only

PRINCIPAL DISPLAY PANEL
- NOVARTIS
- NDC 0078-0386-66
- Myfortic®
- (mycophenolic acid*)
- delayed-release tablets
- *as mycophenolate sodium
- Dispense the accompanying Medication Guide to each patient.
- 120 Tablets
- 360 mg
- Rx only

SRC: NLM .
