Mitapivat
Generic Name:Mitapivat
Brand Name: Pyrukynd
Dosage Formula tablets, intended for oral use
Medically reviewed by S Shah MD. Updated on March 18, 2022.
What is Mitapivat?
Mitapivat is a prescription medication that is used to treat the low count of red blood cells due to the premature break-up of the red blood cell (hemolytic anemia) for adults suffering from pyruvate insufficient kinase (PK Deficiency).
It isn’t known whether Mitapivat is safe and effective for children.
Mitapivat is an active pyruvate kinase, which is present as mitapivat Sulfate. The chemical name of mitapivat sulfate is 8-quinolinesulfonamide, N-[4-[[4- (cyclopropylmethyl)-1-piperazinyl]carbonyl]phenyl]-, sulfate, hydrate (2:1:3). Mitapivat’s chemical formula for is:
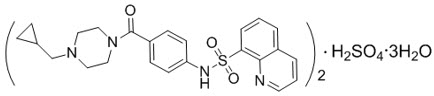
Its molecular formula can be described as (C24H26N4SO3)2 H2SO4 3H2O. it has a molecular weight of 1053.23 for mitapivat Sulfate. Mitapivat sulfur is a white or off-white solid, and it is liquid with water.
This medicine is available in five milligrams, 20 grams, or 50 mg tablets that can be taken orally. Each tablet has five mg, twenty mg, or 50 mg mitapivat-free base, which is provided in the form of 5.85 mg, 23.4 mg, or 58.5 mg, respectively of the salt sulfate hydrate as well as the following active ingredients: croscarmellose salt, microcrystalline cellulose, mannitol, and sodium Stearyl fumarate. ¶
The film coating of the tablet includes inactive ingredients such as FD&C Blue No. 2, hypromellose and lactose monohydrate, titanium dioxide, and triacetin. The tablets are imprinted using black ink that is based on ammonium hydroxide, an inactive ingredient, ferrosoferric oxide alcohol, n-butyl alcohol propylene glycol, and shellac glaze.
Mechanism of Action
Mitapivat is a pyruvate kinase activator that increases pyruvate kinase (PK) activity by allosterically attaching to the pyruvate kinase tetramer. In PK deficiency, the RBC form of pyruvate kinase (PK-R) is altered, resulting in lower adenosine triphosphate (ATP), shorter RBC lifespan, and persistent hemolysis.
Prior to taking Mitapivat
Before starting treatment, inform your physician about any medical issues you have for example if you
- suffer from liver problems.
- Are pregnant or planning to get pregnant. It’s not clear whether Mitapivat can cause harm to your baby. Contact your healthcare professional immediately if you find yourself pregnant or suspect that you’re pregnant during treatment.
- If you are nursing or planning to you plan to. It isn’t known whether Mitapivat gets into the milk. Discuss with your healthcare professional the best method to feed your infant while undergoing treatment.
What other drugs can interact with Mitapivat?
Inform your doctor about the medications you take, which include prescription and over-the-counter medications as well as vitamins and herbal supplements.
Mitapivat along with other medicines can interact, and cause side consequences. Mitapivat could affect how other medicines function and other medications may influence how Mitapivat operates.
Take note of the medications you take. Keep a list to show your healthcare professional or pharmacist when you are prescribed an upcoming medication.
How do I use Mitapivat?
- Take this exactly the way your doctor advises you to do.
- Use the tablets either with or without meals.
- Take the tablets in one swallow. Don’t chew, split or crush the tablets.
- Do not alter your dosage or stop treatment before talking with your doctor. Your doctor will give you the instructions to stop Mitapivat.
- If you have missed the dose by four hours or less you should take your dose as quickly as you can. If more than four days have passed between the regularly scheduled dose, be patient until another dose. You can resume your regular routine for the next dose.¶
Dosing information
Usual Adult Dose for Pyruvate Kinase Deficiency
Start dose: 5 mg taken orally twice daily either with food or no. Check out the below table for dose titration as well as a taper plan.
– Treatment for hemolytic anemia among adults with pyruvate-kinase (PK) deficiency
| Duration | Dosage of medication |
| Week 1 to Week 4 | 5 mg twice a day |
| Week 5 to Week 8 | If Hb is lower than normal levels or if the patient has needed a transfusion in the past 8 weeks:
If Hb is in the normal range and the patient hasn’t needed a transfusion during the past 8 weeks:
|
| Week 9 to Week 12 | If Hb is not within normal levels or the patient needed a transfusion within the past 8 weeks:
If Hb is in the normal range and patient hasn’t required a transfusion during the past 8 weeks:
|
| Maintenance | If Hb levels decrease, think about increasing the dosage to 50 mg twice daily , as according to the schedule above. |
For Missed Dose
If you miss a dosage of Mitapivat by 4 hours or less, take it as soon as possible. Do not provide a replacement dose if a Mitapivat dose is missed by more than 4 hours; instead, wait until the next regular dose. Return to your regular dosing schedule after that.
Interruption or Discontinuation
When feasible, avoid sudden termination or withdrawal of Mitapivat to lessen the risk of acute hemolysis. To gradually stop using the drug, reduce the dose (see TABLE). Keep an eye on patients for signs of acute hemolysis and anemia worsening.
| Current Dose | Dose Taper Schedule | ||
|---|---|---|---|
| Day 1-7 | Day 8-14 | Day 15 | |
| Abbreviations: N/A = not applicable. | |||
| 5 mg twice daily | 5 mg once daily | Discontinue | N/A |
| 20 mg twice daily | 20 mg once daily | 5 mg once daily | Discontinue |
| 50 mg twice daily | 50 mg once daily | 20 mg once daily | Discontinue |
Mitapivat side effects
Mitapivat could be a cause of serious adverse reactions, including:
- The rapid break-up of the red blood cells (acute hemolysis) This has happened following a sudden interruption or discontinuing treatment. Do not abruptly stop treatment with Mitapivat. If you must stop treatment, your doctor will closely watch you. Contact your healthcare professional immediately if you experience any symptoms or signs of the breakdown of red blood cells, including:
- Skin yellowing or the eyes’ whites (jaundice)
- Urine that is dark in color
- dizziness
- hives
- confusion
- Feeling tired
- breathlessness
- chest pain
The most commonly reported adverse effects are:
- reduction in the hormone that regulates reproduction (testosterone) in males
- more salt as a result of uric acids (urate) the blood test
- reduction in the hormone of reproduction (estradiol) in males
- joint discomfort (arthralgia)
- Back discomfort
This is not the only list of the possible adverse negative effects. Consult your physician for advice from a medical professional regarding adverse effects. You can report adverse reactions to the FDA at 1-800-FDA-1088.
How do I store it?
- Keep it at room temperature , between 68degF and 77degF (20degC up to 25degC).
- Place the pockets in their original packaging until they are used.
General information regarding the safety
Sometimes, medicines are used for reasons other than those described in the Patient Information leaflet. Use this medication only to treat a problem that was not recommended. Don’t give it to others even if they suffer from the same symptoms as you do. It could harm them. You may seek advice from your doctor or pharmacist for advice designed for health professionals.
How Supplied
| Tablet Strength | Description | Imprint | NDC |
|---|---|---|---|
| 5 mg | Round, blue, film-coated tablets | “M5” printed on one side | 71334-205-05 |
| 20 mg | Round, blue, film-coated tablets | “M20” printed on one side | 71334-210-20 |
| 50 mg | Oblong, blue, film-coated tablets | “M50” printed on one side | 71334-215-50 |
| Tablet strength(s) | Blister Wallet Configuration | Tablet Description | Imprint | NDC |
|---|---|---|---|---|
| 5 mg |
|
round, blue, film-coated tablets | “M5” printed on one side | 71334-220-11 |
| 20 mg and 5 mg |
|
round, blue, film-coated tablets | “M20” printed on one side | 71334-225-12 |
|
round, blue, film-coated tablets | “M5” printed on one side | ||
| 50 mg and 20 mg |
|
oblong, blue, film-coated tablets | “M50” printed on one side | 71334-230-13 |
|
round, blue, film-coated tablets | “M20” printed on one side |
Label
5 mg Tab
- Mitapivat tablets
5 mg per tablet - 56 tablets
- Contains 4-week supply of Mitapivat
(Four 7-day blister wallets with 14 tablets per wallet)
Swallow the tablets whole. Do Not split, crush, chew, or dissolve the tablets.

20 mg Tab
- Mitapivat tablets
20 mg per tablet - 56 tablets
- Contains a 4-week supply of Mitapivat
(Four 7-day blister wallets with 14 tablets per wallet)
Swallow the tablets whole. Do Not split, crush, chew, or dissolve the tablets.

50 mg Tab
- Mitapivat tablets
50 mg per tablet - 56 tablets
- Contains 4-week supply of Mitapivat
(Four 7-day blister wallets with 14 tablets per wallet)
Swallow tablets whole. Do Not split, crush, chew, or dissolve the tablets.

More details
Always consult your physician to make sure the information presented on this site is appropriate to your specific situation.
