Mirapex ER
Generic name: pramipexole
Brand names: Mirapex, Mirapex ER
Drug class: Dopaminergic antiparkinsonism agents
Medically reviewed by A Ras MD.
What is Mirapex ER?
Mirapex ER is a prescription medicine used to treat the signs and symptoms of Parkinson’s disease. It is not known if Mirapex ER is safe and effective in children.
Description
MIRAPEX ER tablets contain pramipexole dihydrochloride (as a monohydrate). Pramipexole is a non-ergot dopamine agonist. The chemical name of pramipexole dihydrochloride monohydrate is (S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate. Its empirical formula is C10 H17 N3 S ∙ 2HCl ∙ H2O, and its molecular weight is 302.26.
The structural formula is:
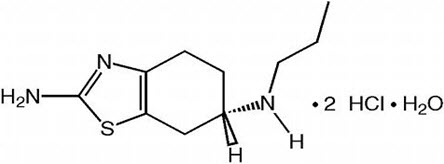
Pramipexole dihydrochloride is a white to off-white powder substance. Melting occurs in the range of 296°C to 301°C, with decomposition. Pramipexole dihydrochloride is more than 20% soluble in water, about 8% in methanol, about 0.5% in ethanol, and practically insoluble in dichloromethane.
MIRAPEX ER tablets 0.375 mg:
Each extended-release tablet contains 0.375 mg pramipexole dihydrochloride monohydrate equivalent to 0.352 mg pramipexole dihydrochloride.
MIRAPEX ER tablets 0.75 mg:
Each extended-release tablet contains 0.75 mg pramipexole dihydrochloride monohydrate equivalent to 0.705 mg pramipexole dihydrochloride.
MIRAPEX ER tablets 1.5 mg:
Each extended-release tablet contains 1.5 mg pramipexole dihydrochloride monohydrate equivalent to 1.41 mg pramipexole dihydrochloride.
MIRAPEX ER tablets 2.25 mg:
Each extended-release tablet contains 2.25 mg pramipexole dihydrochloride monohydrate equivalent to 2.12 mg pramipexole dihydrochloride.
MIRAPEX ER tablets 3 mg:
Each extended-release tablet contains 3 mg pramipexole dihydrochloride monohydrate equivalent to 2.82 mg pramipexole dihydrochloride.
MIRAPEX ER tablets 3.75 mg:
Each extended-release tablet contains 3.75 mg pramipexole dihydrochloride monohydrate equivalent to 3.53 mg pramipexole dihydrochloride.
MIRAPEX ER tablets 4.5 mg:
Each extended-release tablet contains 4.5 mg pramipexole dihydrochloride monohydrate equivalent to 4.23 mg pramipexole dihydrochloride.
Inactive ingredients for all strengths of MIRAPEX ER tablets consist of hypromellose, corn starch, carbomer homopolymer, colloidal silicon dioxide, and magnesium stearate.
Mechanism of Action
Pramipexole is a non-ergot dopamine agonist with high relative in vitro specificity and full intrinsic activity at the D2 subfamily of dopamine receptors, binding with higher affinity to D3 than to D2 or D4 receptor subtypes.
The precise mechanism of action of pramipexole as a treatment for Parkinson’s disease is unknown, although it is believed to be related to its ability to stimulate dopamine receptors in the striatum. This conclusion is supported by electrophysiologic studies in animals that have demonstrated that pramipexole influences striatal neuronal firing rates via activation of dopamine receptors in the striatum and the substantia nigra, the site of neurons that send projections to the striatum. The relevance of D3 receptor binding in Parkinson’s disease is unknown.
What should I tell my healthcare provider before taking Mirapex ER?
Before taking Mirapex ER, tell your doctor if you:
- feel sleepy during the day
- have low blood pressure, or if you feel dizzy or faint, especially when getting up from sitting or lying down.
- have trouble controlling your muscles (dyskinesia)
- have kidney problems
- drink alcohol. Alcohol can increase the chance that Mirapex ER will make you feel sleepy or fall asleep when you should be awake.
- are pregnant or plan to become pregnant. It is not known if Mirapex ER will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Mirapex ER passes into your breast milk. You and your doctor should decide if you will take Mirapex ER or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Mirapex ER and other medicines may affect each other causing side effects. Mirapex ER may affect the way other medicines work, and other medicines may affect how Mirapex ER works.
Especially tell your doctor if you take:
- medicines called neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide. Mirapex ER may not work as well if you take these medicines.
- pramipexole (Mirapex). Pramipexole is the active ingredient in both Mirapex ER and Mirapex. If you are taking Mirapex, you should not take Mirapex ER.
- any other medicines that make you sleepy or may increase the effects of Mirapex ER, such as cimetidine (Tagamet).
Ask your doctor for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
How should I take Mirapex ER?
- Mirapex ER is taken once daily.
- Your doctor will tell you how much Mirapex ER to take and when to take it. Do not take more or less Mirapex ER than your doctor tells you to.
- Swallow Mirapex ER whole. Do not chew, crush, or cut Mirapex ER.
- Mirapex ER can be taken with or without food. Taking Mirapex ER with food may lower your chances of getting nausea.
- You may see something that looks like a swollen original tablet or swollen pieces of the original tablet in your stool. If this happens, tell your doctor.
- If you miss a dose of Mirapex ER it should be taken as soon as possible, but no later than 12 hours after your regularly scheduled time. If it is later than 12 hours, the missed dose should be skipped and the next dose should be taken on the following day at your regularly scheduled time. Do not double your next Mirapex ER dose.
- Do not stop taking Mirapex ER without talking to your doctor first. If your doctor tells you to stop taking Mirapex ER, you should ask your doctor for specific instructions on how to slowly and safely discontinue taking Mirapex ER. If you stop taking Mirapex ER too quickly you may have withdrawal symptoms such as:
- fever
- confusion
- severe muscle stiffness
What should I avoid while taking Mirapex ER?
- Do not drink alcohol while taking Mirapex ER. It can increase your chance of having serious side effects. See “What are the possible side effects of Mirapex ER?”
- Do not drive a car, operate a machine, or do other dangerous activities until you know how Mirapex ER affects you. Sleepiness caused by Mirapex ER can happen as late as 1 year after you start your treatment.
What are the possible side effects of Mirapex ER?
Mirapex ER may cause serious side effects, including:
- falling asleep during normal daily activities. Mirapex ER may cause you to fall asleep while you are doing daily activities such as driving, talking with other people, or eating.
- Some people taking the medicine in Mirapex ER have had car accidents because they fell asleep while driving.
- Some people did not feel sleepy before they fell asleep while driving. You could fall asleep without any warning.
Tell your doctor right away if you fall asleep while you are doing activities such as talking, eating, driving, or if you feel sleepier than normal for you.
- low blood pressure when you sit or stand up quickly. After you have been sitting or lying down, stand up slowly until you know how Mirapex ER affects you. This may help reduce the following symptoms while you are taking Mirapex ER:
- unusual urges. Some people who take certain medicines to treat Parkinson’s disease, including Mirapex ER, have reported problems, such as gambling, compulsive eating, compulsive buying, and increased sex drive.
If you or your family members notice that you are developing unusual urges or behaviors, talk to your doctor. - hallucinations and other psychotic-like behavior (seeing visions, hearing sounds or feeling sensations that are not real, confusion, excessive suspicion, aggressive behavior, agitation, delusional beliefs and disorganized thinking). Your chance of having hallucinations and other psychotic-like behavior is higher if you are age 65 or older.
If you have hallucinations or other psychotic-like changes, talk with your doctor right away. - uncontrolled sudden movements (dyskinesia). If you have new dyskinesia, or your existing dyskinesia gets worse, tell your doctor.
- posture changes. Talk with your doctor if you have posture changes you cannot control. These may include your neck bending forward, bending forward at the waist, or tilting sideways when you sit, stand, or walk.
The most common side effects in people taking Mirapex ER for early Parkinson’s disease are:
- nausea and vomiting
- constipation
- dizziness
- fatigue
- dry mouth
- swelling of the feet and ankles
The most common side effects in people taking Mirapex ER who have later stage Parkinson’s disease are nausea, constipation, headache and weight loss (anorexia).
These are not all the possible side effects of Mirapex ER. Tell your doctor if you have any side effect that bothers you.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Mirapex ER
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Mirapex ER for a condition for which it was not prescribed. Do not give Mirapex ER to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Mirapex ER. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for more information about Mirapex ER tablets that is written for healthcare professionals.
For current Prescribing Information call Boehringer Ingelheim Pharmaceuticals, Inc. at 1-800-542-6257 or 1-800-459-9906 TTY.
How should I store Mirapex ER?
- Store Mirapex ER at room temperature from 68°F to 77°F (20°C to 25°C).
- Keep Mirapex ER away from high humidity or moisture.
- Keep Mirapex ER and all medicines out of the reach of children.
What are the ingredients in Mirapex ER?
Active Ingredient: pramipexole dihydrochloride monohydrate.
Inactive Ingredients: hypromellose, corn starch, carbomer homopolymer, colloidal silicon dioxide, and magnesium stearate.
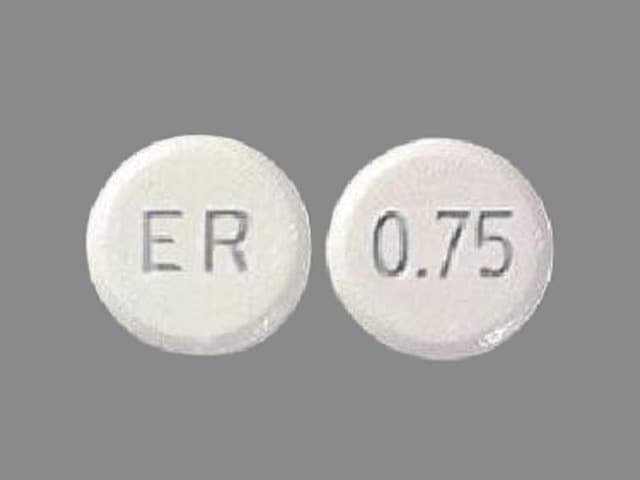
What does Mirapex ER look like?
These pictures show what Mirapex ER tablets look like. Notice that each strength tablet looks different. Immediately call your pharmacist if you receive a Mirapex ER tablet that does not look like one of the tablets shown below, as you may have received the wrong medication.
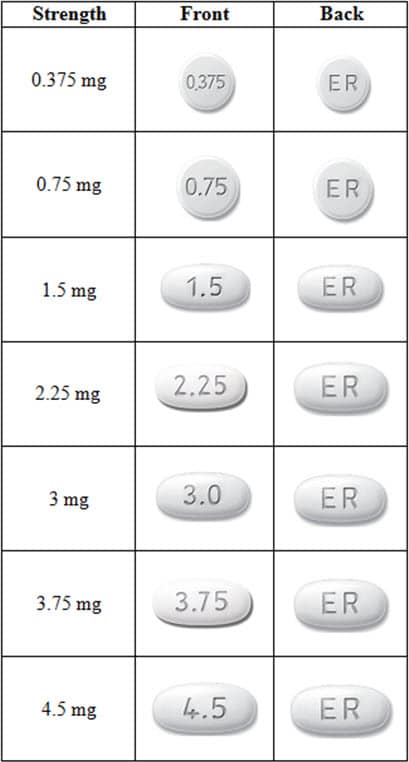
Tablets not actual size.
Label
PRINCIPAL DISPLAY PANEL – 0.375 MG TABLET BOTTLE LABEL
- NDC 0597-0109-30
30 Tablets - Mirapex ER®
(pramipexole dihydrochloride
extended-release tablets) - 0.375 mg*
Once Daily - Dispense in this ORIGINAL Unit of Use Container
- Rx only
- Boehringer
Ingelheim

PRINCIPAL DISPLAY PANEL – 0.75 MG TABLET BOTTLE LABEL
- NDC 0597-0285-30
30 Tablets - Mirapex ER®
(pramipexole dihydrochloride
extended-release tablets) - 0.75 mg*
Once Daily - Dispense in this ORIGINAL Unit of Use Container
- Rx only
- Boehringer
Ingelheim

PRINCIPAL DISPLAY PANEL – 1.5 MG TABLET BOTTLE LABEL
- NDC 0597-0113-30
30 Tablets - Mirapex ER®
(pramipexole dihydrochloride
extended-release tablets) - 1.5 mg*
Once Daily - Dispense in this ORIGINAL Unit of Use Container
- Rx only
- Boehringer
Ingelheim


PRINCIPAL DISPLAY PANEL – 2.25 MG TABLET BOTTLE LABEL
- NDC 0597-0286-30
30 Tablets - Mirapex ER®
(pramipexole dihydrochloride
extended-release tablets) - 2.25 mg*
Once Daily - Dispense in this ORIGINAL Unit of Use Container
- Rx only
- Boehringer
Ingelheim


PRINCIPAL DISPLAY PANEL – 3 MG TABLET BOTTLE LABEL
- NDC 0597-0115-30
30 Tablets - Mirapex ER®
(pramipexole dihydrochloride
extended-release tablets) - 3 mg*
Once Daily - Dispense in this ORIGINAL Unit of Use Container
- Rx only
- Boehringer
Ingelheim

PRINCIPAL DISPLAY PANEL – 4.5 MG TABLET BOTTLE LABEL
- NDC 0597-0116-30
30 Tablets - Mirapex ER®
(pramipexole dihydrochloride
extended-release tablets) - 4.5 mg*
Once Daily - Dispense in this ORIGINAL Unit of Use Container
- Rx only
- Boehringer
Ingelheim


SRC: NLM .