Menostar
Generic name: estradiol transdermal
Drug class: Estrogens
Medically reviewed by A Ras MD.
What is Menostar?
Menostar is a prescription medicine patch (Transdermal System) that contains estradiol (an estrogen hormone).
Description
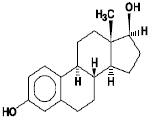
Menostar (estradiol transdermal system) is designed to provide nominal in vivo delivery of 14 mcg of estradiol per day continuously upon application to intact skin. The period of use is 7 days. The transdermal system has a contact surface area of 3.25 cm2, and contains 1 mg of estradiol USP.
Estradiol USP is a white, crystalline powder, chemically described as estra-1,3,5(10)-triene-3, 17ß-diol. It has an empirical formula of C18H24O2 and molecular weight of 272.38. The structural formula is:
The Menostar transdermal system comprises three layers. Proceeding from the visible surface toward the surface attached to the skin, these layers are:
1. A translucent polyethylene film.
2. An acrylate adhesive matrix containing estradiol USP.
3. A protective liner of siliconized or fluoropolymer-coated polyester film is attached to the adhesive surface and must be removed before the transdermal system can be used.
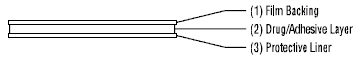
The active component of the transdermal system is estradiol. The remaining components of the transdermal system (acrylate copolymer adhesive, fatty acid esters, and polyethylene backing) are pharmacologically inactive.
What is Menostar used for?
Menostar is used after menopause to:
- Help reduce your chances of getting osteoporosis (thin weak bones)
- If you use Menostar only to prevent osteoporosis due to menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you.
Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, which is secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
What is the most important information I should know about Menostar?
- Using estrogen-alone may increase your chance of getting cancer of the uterus (womb). Report any unusual vaginal bleeding right away while you are using Menostar. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- Do not use estrogen-alone to prevent heart disease, heart attacks, strokes, or dementia (decline in brain function).
- Using estrogen-alone may increase your chances of getting strokes or blood clots.
- Using estrogen-alone may increase your chance of getting dementia, based on a study of women age 65 years of age or older.
- Do not use estrogens with progestins to prevent heart disease, heart attacks, strokes or dementia.
- Using estrogens with progestins may increase your chances of getting heart attacks, strokes, breast cancer, or blood clots.
- Using estrogens with progestins may increase your chance of getting dementia, based on a study of women age 65 years of age or older.
- You and your healthcare provider should talk regularly about whether you still need treatment with Menostar.
Who should not use Menostar?
Do not start using Menostar if you:
- have unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause. - currently have or have had certain cancers
Estrogens may increase the chance of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should use Menostar. - had a stroke or heart attack
- currently have or have had blood clots
- currently have or have had liver problems
- have been diagnosed with a bleeding disorder
- are allergic to Menostar or any of its ingredients
See the list of ingredients in Menostar at the end of this leaflet. - think you may be pregnant
Menostar is not for pregnant women. If you think you may be pregnant, you should have a pregnancy test and know the results. Do not use Menostar if the test is positive and talk to your healthcare provider.
What should I tell my healthcare provider before using Menostar?
Before you use Menostar, tell your healthcare provider if you:
- have any unusual vaginal bleeding
Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause. - have any other medical conditions
Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, angioedema (swelling of face and tongue), or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood. - are going to have surgery or will be on bed rest
Your healthcare provider will let you know if you need to stop using Menostar. - are breastfeeding
The hormone in Menostar can pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how Menostar works. Menostar may also affect how your other medicines work. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use Menostar?
For detailed instructions, see the step-by-step instructions that come with Menostar.
- Use Menostar exactly as your healthcare provider tells you to use it.
- Menostar is for skin use only.
- Change your Menostar patch 1 time each week or every 7 days.
- Apply your Menostar patch to a clean, dry area on your lower abdomen or buttocks. This area must be clean, dry, and free of powder, oil or lotion for your patch to stick to your skin.
- Apply your Menostar patch to a different area of your abdomen or your buttocks each time. Do not use the same application site 2 times in the same week.
- Do not apply Menostar to your breasts.
- If you forget to apply a new Menostar patch, you should apply a new patch as soon as possible.
- You and your healthcare provider should talk regularly (every 3 to 6 months) about the dose you are using and whether you still need treatment with Menostar.
How to Change Menostar.
- When changing Menostar, peel off the used patch slowly from the skin.
- After removal of Menostar, people usually have either no adhesive residue or light adhesive residue. If any adhesive residue remains on your skin after removing the patch, allow the area to dry for 15 minutes. Then, gently rub the area with an oil-based cream or lotion to remove the adhesive from your skin.
- Keep in mind, the new patch must be applied to a different skin area of your abdomen or buttocks. This area must be clean, dry, and free of powder, oil or lotion. The same site should not be used again for at least 1 week after removal of the patch.
What are the possible side effects of Menostar?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious, but less common side effects include:
- heart attack
- stroke
- blood clots
- dementia
- breast cancer
- cancer of the lining of the uterus (womb)
- cancer of the ovary
- high blood pressure
- high blood sugar
- gallbladder disease
- liver problems
- changes in your thyroid hormone levels
- enlargement of benign tumors of the uterus (“fibroids”)
Call your healthcare provider right away if you get any of the following warning signs or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
- Less serious, but common side effects include:
- headache
- breast tenderness or pain
- irregular vaginal bleeding or spotting
- stomach or abdominal cramps, bloating
- nausea and vomiting
- hair loss
- fluid retention
- vaginal yeast infection
- redness or irritation at the patch placement site
These are not all the possible side effects of Menostar. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effects that bother you or does not go away.
You may report side effects to Bayer Healthcare Pharmaceuticals at 1-888-842-2937 or to FDA at 1-800-FDA-1088.
What can I do to lower my chances of a serious side effect with Menostar?
- Talk with your healthcare provider regularly about whether you should continue using Menostar.
- If you have a uterus, talk with your healthcare provider about whether the addition of a progestin is right for you.
- The addition of a progestin is generally recommended for women with a uterus to reduce the chance of getting cancer of the uterus (womb).
- See your healthcare provider right away if you get vaginal bleeding while using Menostar.
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else.
- If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances of getting heart disease.
Ask your healthcare provider for ways to lower your chances of getting heart disease.
General information about the safe and effective use of Menostar
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use Menostar for conditions for which it was not prescribed. Do not give Menostar to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Menostar. If you would like more information, talk with your healthcare provider or pharmacist. You can ask for information about Menostar that is written for health professionals.
For more information, go to WWW.MENOSTAR-US.COM or call Bayer Healthcare Pharmaceuticals Inc. at 1-888-842-2937.
How should I store Menostar?
- Store Menostar at room temperature 68°F to 77°F (20°C to 25°C).
- Do not store Menostar patches outside of their pouches. Apply immediately upon removal from the protective pouch.
- Used patches still contain estrogen. To throw away the patch, fold the sticky side of the patch together, place it in a sturdy child-proof container, and place this container in the trash. Used patches should not be flushed in the toilet.
Keep Menostar and all medicines out of the reach of children.
What are the ingredients in Menostar?
Active ingredient: estradiol
Inactive ingredients: acrylate copolymer adhesive, fatty acid esters, and polyethylene backing.
Label
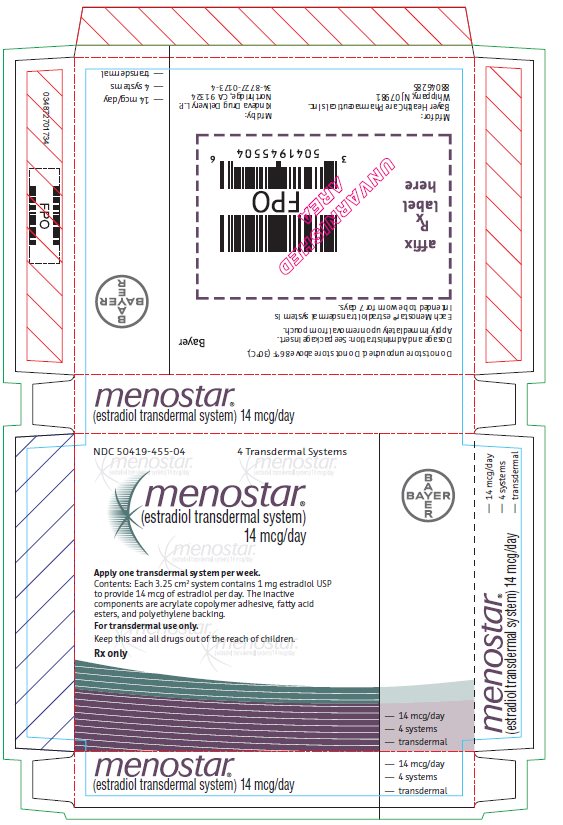
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- Menostar Individual Carton
- NDC 50419-455-04 4 Transdermal Systems
- Menostar
- (estradiol transdermal system)
14 mcg/day - Contents: Each 3.25 cm2 system contains 1 mg estradiol
USP to provide 14 mcg of estradiol per day. The inactive
components are acrylate copolymer adhesive, fatty acid
esters, and polyethylene backing. - For Transdermal Use Only.
- Keep this and all drugs out of the reach of children.
- Rx Only
SRC: NLM .
