Lyrica CR
Generic name: pregabalin
Dosage forms: oral tablet, extended release (82.5 mg: 165 mg; 330 mg;
Drug class: Gamma-aminobutyric acid analogs
Medically reviewed by A Ras MD.
What is Lyrica CR?
Lyrica CR is a prescription medicine used treat pain from damaged nerves (neuropathic pain) that happens with diabetes. It is also used in the treatment of pain from damaged nerves (neuropathic pain) that follows healing of shingles
It is not known if Lyrica CR is safe and effective in children.
It is not known if Lyrica CR is effective when used for the treatment of fibromyalgia, or when taken with other seizure medicines for adults with partial onset seizures.
Description
LYRICA CR (pregabalin extended-release) tablets are for oral use and contain pregabalin. Pregabalin is described chemically as (S)-3-(aminomethyl)-5-methylhexanoic acid. The molecular formula is C8H17NO2 and the molecular weight is 159.23. The chemical structure of pregabalin is:
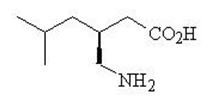
Pregabalin is a white to off-white, crystalline solid with a pKa1 of 4.2 and a pKa2 of 10.6. It is freely soluble in water and both basic and acidic aqueous solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is – 1.35.
LYRICA CR extended-release tablets are administered orally and contain 82.5, 165, or 330 mg of pregabalin, along with Kollidon SR (polyvinyl acetate, povidone, sodium lauryl sulphate, and silica), crospovidone, polyethylene oxide, carbomer, magnesium stearate, polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol, and colorants as inactive ingredients.
Mechanism of Action
Pregabalin binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in central nervous system tissues. Although the mechanism of action of pregabalin has not been fully elucidated, results with genetically modified mice and with compounds structurally related to pregabalin (such as gabapentin) suggest that binding to the alpha2-delta subunit may be involved in pregabalin’s anti-nociceptive and antiseizure effects in animals. In animal models of nerve damage, pregabalin has been shown to reduce calcium-dependent release of pro-nociceptive neurotransmitters in the spinal cord, possibly by disrupting alpha2-delta containing-calcium channel trafficking and/or reducing calcium currents. Evidence from other animal models of nerve damage and persistent pain suggest the anti-nociceptive activities of pregabalin may also be mediated through interactions with descending noradrenergic and serotonergic pathways originating from the brainstem that modulate pain transmission in the spinal cord.
While pregabalin is a structural derivative of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA), it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, does not augment GABAA responses in cultured neurons, does not alter rat brain GABA concentration or have acute effects on GABA uptake or degradation. However, in cultured neurons prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transport. Pregabalin does not block sodium channels, is not active at opiate receptors, and does not alter cyclooxygenase enzyme activity. It is inactive at serotonin and dopamine receptors and does not inhibit dopamine, serotonin, or noradrenaline reuptake.
What is the most important information I should know about Lyrica CR?
Lyrica CR may cause serious side effects including:
- Serious, even life-threatening, allergic reactions
- Suicidal thoughts or actions
- Swelling of your hands, legs and feet
- Dizziness and sleepiness
These serious side effects are described below:
- Serious, even life-threatening, allergic reactions.
Stop taking Lyrica CR and call your healthcare provider right away if you have any of these signs of a serious allergic reaction:- swelling of your face, mouth, lips, gums, tongue, throat, or neck
- trouble breathing
- rash, hives (raised bumps), or blisters
- skin redness
- Lyrica CR may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- trouble sleeping (insomnia)
- new or worse irritability
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or moodIf you have suicidal thoughts or actions, do not stop Lyrica CR without first talking to a healthcare provider.
- Stopping Lyrica CR suddenly can cause serious problems.
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
- Serious breathing problems can occur when Lyrica is taken with other medicines that can cause severe sleepiness or decreased awareness, or when it is taken by someone who already has breathing problems. Watch for increased sleepiness or decreased breathing when starting Lyrica or when the dose is increased. Get help right away if breathing problems occur.
- Swelling of your hands, legs and feet. This swelling can be a serious problem for people with heart problems.
- Dizziness and sleepiness. Do not drive a car, work with machines, or do other dangerous activities until you know how Lyrica CR affects you. Ask your healthcare provider about when it will be okay to do these activities.
Who should not take Lyrica CR?
Do not take Lyrica CR if you are allergic to pregabalin or any of the ingredients in Lyrica CR.
See “What is the most important information I should know about Lyrica CR?” for the signs of an allergic reaction.
See the end of this leaflet for a complete list of ingredients in Lyrica CR.
What should I tell my healthcare provider before taking Lyrica CR?
Before taking Lyrica CR, tell your healthcare provider about all your medical conditions, including if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have kidney problems or get kidney dialysis
- have heart problems including heart failure
- have a bleeding problem or a low blood platelet count
- have abused prescription medicines, street drugs, or alcohol in the past
- have ever had swelling of your face, mouth, tongue, lips, gums, neck, or throat (angioedema)
- plan to father a child. Animal studies have shown that pregabalin, the active ingredient in Lyrica CR, made male animals less fertile and caused sperm to change. Also, in animal studies, birth defects were seen in the offspring (babies) of male animals treated with pregabalin. It is not known if these problems can happen in people who take Lyrica CR.
- are pregnant or plan to become pregnant. It is not known if Lyrica CR will harm your unborn baby. You and your healthcare provider will have to decide if you should take Lyrica CR while you are pregnant.
- If you become pregnant while taking Lyrica CR, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs, including pregabalin, the active ingredient in Lyrica CR. Information about the registry can be found at the website, http://www.aedpregnancyregistry.org/.
- are breastfeeding or plan to breastfeed. Lyrica CR passes into your breast milk. It is not known if Lyrica CR can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take Lyrica CR. Breastfeeding is not recommended while taking Lyrica CR.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins or herbal supplements. Lyrica CR and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take:
- angiotensin converting enzyme (ACE) inhibitors, which are used to treat many conditions, including high blood pressure. You may have a higher chance for swelling and hives if these medicines are taken with Lyrica CR. See “What is the most important information I should know about Lyrica CR?”
- Avandia (rosiglitazone), Avandamet (contains rosiglitazone and metformin), or Actos (pioglitazone) for diabetes. You may have a higher chance of weight gain or swelling of your hands or feet if these medicines are taken with Lyrica CR. See “What are the possible side effects of Lyrica CR?.”
- any opioid pain medicine (such as oxycodone), or medicines for anxiety (such as lorazepam) or insomnia (such as zolpidem). You may have a higher chance for dizziness, sleepiness or serious breathing problems if these medicines are taken with Lyrica CR.
- any medicines that make you sleepy
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist each time you get a new medicine. Do not start a new medicine without talking with your healthcare provider.
How should I take Lyrica CR?
- Take Lyrica CR exactly as prescribed. Your healthcare provider will tell you how much Lyrica CR to take and when to take it.
- Take Lyrica CR at the same time each day.
- Lyrica CR must be taken after your evening meal. Swallow the tablet whole and do not split, crush or chew the tablet.
- Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
- Do not stop taking Lyrica CR without talking to your healthcare provider. If you stop taking Lyrica CR suddenly you may have headaches, nausea, diarrhea, trouble sleeping, or you may feel anxious. If you have epilepsy, are taking Lyrica CR for pain, and stop taking Lyrica CR suddenly, you may have seizures more often. Talk with your healthcare provider about how to stop Lyrica CR slowly.
- If you miss a dose after your evening meal, take it prior to bedtime following a snack. If you miss the dose prior to bedtime, then take it following your morning meal. If you do not take the dose the following morning, then take the next dose at your regular time after your evening meal. Do not take 2 doses at the same time.
- If you take too much Lyrica CR, call your healthcare provider or poison control center, or go to the nearest emergency room right away.
What should I avoid while taking Lyrica CR?
- Do not drive a car, work with machines, or do other dangerous activities until you know how Lyrica CR affects you.
- Do not drink alcohol while taking Lyrica CR. Lyrica CR and alcohol can affect each other and increase side effects such as sleepiness and dizziness.
What are the possible side effects of Lyrica CR?
Lyrica CR may cause serious side effects, including:
- muscle problems, muscle pain, soreness, or weakness. If you have these symptoms, especially if you feel sick and have a fever, tell your healthcare provider right away.
- problems with your eyesight, including blurry vision. Call your healthcare provider if you have any changes in your eyesight.
- weight gain. If you have diabetes, weight gain may affect the management of your diabetes. Weight gain can also be a serious problem for people with heart problems.
- Feeling “high”
The most common side effects of Lyrica CR are:
- dizziness
- blurry vision
- weight gain
- sleepiness
- fatigue (tiredness)
- swelling of hands and feet
- dry mouth
- nausea
Lyrica CR caused skin sores in animal studies. Skin sores did not happen in studies in people. If you have diabetes, you should pay attention to your skin while taking Lyrica CR and tell your healthcare provider about any sores or skin problems.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of Lyrica CR. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Lyrica CR
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Lyrica CR for a condition for which it was not prescribed. Do not give Lyrica CR to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacists or healthcare provider for information about Lyrica CR that is written for health professionals.
You can also visit the Lyrica CR website at www.LYRICA.com or call 1-866-459-7422 (1-866-4LYRICA).
How should I store Lyrica CR?
- Store Lyrica CR at room temperature between 68°F to 77°F (20°C to 25°C) in its original package.
- Safely throw away any Lyrica CR that is out of date or no longer needed.
Keep Lyrica CR and all medicines out of the reach of children.
What are the ingredients in Lyrica CR?
Active ingredient: pregabalin
Inactive ingredients: Kollidon SR (polyvinyl acetate, povidone, sodium lauryl sulphate, and silica), crospovidone, polyethylene oxide, carbomer, magnesium stearate, polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol, and colorants.
Label
PRINCIPAL DISPLAY PANEL – 82.5 MG TABLET BOTTLE LABEL
- Pfizer
NDC 0071-1026-01 - Lyrica® CR
(pregabalin)
extended release tablets - CV
- 82.5 mg
- ALWAYS DISPENSE WITH
MEDICATION GUIDE - 30 Tablets
Rx only

PRINCIPAL DISPLAY PANEL – 165 MG TABLET BOTTLE LABEL
- Pfizer
NDC 0071-1027-01 - Lyrica® CR
(pregabalin)
extended release tablets - CV
- 165 mg
- ALWAYS DISPENSE WITH
MEDICATION GUIDE - 30 Tablets
Rx only 
PRINCIPAL DISPLAY PANEL – 330 MG TABLET BOTTLE LABEL
- Pfizer
NDC 0071-1029-01 - Lyrica® CR
(pregabalin)
extended release tablets - CV
- 330 mg
- ALWAYS DISPENSE WITH
MEDICATION GUIDE - 30 Tablets
Rx only

SRC: NLM .
