Lunesta
Generic name: eszopiclone
Drug class: Miscellaneous anxiolytics, sedatives and hypnotics
Medically reviewed by A Ras MD.
What is Lunesta?
Lunesta is a sedative-hypnotic (sleep) medicine. Lunesta is used in adults for the treatment of a sleep problem called insomnia. Symptoms of insomnia include trouble falling asleep, waking up often during the night
Lunesta is not for children.
| Lunesta is a federally controlled substance (C-IV) because it can be abused or lead to dependence. Keep Lunesta in a safe place to prevent misuse and abuse. Selling or giving away Lunesta may harm others, and is against the law. Tell your doctor if you have ever abused or been dependent on alcohol, prescription medicines or street drugs. |
Description
LUNESTA (eszopiclone) is a nonbenzodiazepine hypnotic agent that is a pyrrolopyrazine derivative of the cyclopyrrolone class. The chemical name of eszopiclone is (+)-(5S)-6-(5-chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazin-5-yl 4-methylpiperazine-1-carboxylate. Its molecular weight is 388.81, and its empirical formula is C17H17ClN6O3. Eszopiclone has a single chiral center with an (S)-configuration. It has the following chemical structure:

Eszopiclone is a white to light-yellow crystalline solid. Eszopiclone is very slightly soluble in water, slightly soluble in ethanol, and soluble in phosphate buffer (pH 3.2).
Eszopiclone is formulated as film-coated tablets for oral administration. LUNESTA tablets contain 1 mg, 2 mg, or 3 mg eszopiclone and the following inactive ingredients: calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and triacetin. In addition, both the 1 mg and 3 mg tablets contain FD&C Blue #2.
Mechanism of Action
The mechanism of action of eszopiclone as a hypnotic is unclear; however, its effect could be related to its interaction with GABA-receptor complexes at binding domains located close to or allosterically coupled to benzodiazepine receptors.
What is the most important information I should know about Lunesta?
- Do not take more Lunesta than prescribed.
- Do not take Lunesta unless you are able to stay in bed a full night (7 to 8 hours) before you must be active again.
- Take Lunesta right before you get in bed, not sooner.
Lunesta may cause serious side effects, including:
Complex sleep behaviors that have caused serious injury and death. After taking Lunesta, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing (complex sleep behaviors). The next morning, you may not remember that you did anything during the night. These activities may occur with Lunesta whether or not you drink alcohol or take other medicines that make you sleepy.
Reported activities and behaviors include:
- doing activities when you are asleep like:
- making and eating food
- talking on the phone
- having sex
- driving a car (“sleep-driving”)
- sleep walking
Stop taking Lunesta and call your healthcare provider right away if you find out that you have done any of the above activities after taking Lunesta.
The morning after you take Lunesta your ability to drive safely and think clearly may be decreased. You also may experience sleepiness during the day.
Do not take Lunesta if you:
- have ever experienced a complex sleep behavior (such as driving a car, making and eating food, talking on the phone or having sex while not fully awake) after taking Lunesta
- drank alcohol that evening or before bed
- take other medicines that can make you sleepy. Talk to your doctor about all of your medicines. Your doctor will tell you if you can take Lunesta with your other medicines.
- cannot get a full night’s sleep
Who should not take Lunesta?
- Do not take Lunesta if you have ever had a complex sleep behavior happen after taking Lunesta.
- Do not take Lunesta if you are allergic to anything in it. See the end of this Medication Guide for a complete list of ingredients in Lunesta.
What should I tell my healthcare provider before taking Lunesta?
Lunesta may not be right for you. Before starting Lunesta, tell your healthcare provider about all of your health conditions, including if you:
- have a history of depression, mental illness, or suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- have liver disease
- are pregnant, planning to become pregnant, or breastfeeding
Tell your healthcare provider about all of the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Medicines can interact with each other, sometimes causing serious side effects. Do not take Lunesta with other medicines that can make you sleepy.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take Lunesta?
- Take Lunesta exactly as prescribed. Do not take more Lunesta than prescribed for you.
- Take Lunesta right before you get into bed.
- Do not take Lunesta with or right after a meal.
- Do not take Lunesta unless you are able to get a full night’s sleep before you must be active again.
- Call your healthcare provider if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problems.
- If you take too much Lunesta or overdose, call your healthcare provider or poison control center right away, or get emergency treatment.
What are the possible side effects of Lunesta?
Possible serious side effects of Lunesta include:
- getting out of bed while not being fully awake and doing an activity that you do not know you are doing. (See “What is the most important information I should know about Lunesta?”)
- abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, acting strangely, hallucinations, worsening of depression, and suicidal thoughts or actions.
- memory loss
- anxiety
- severe allergic reactions. Symptoms include swelling of the tongue or throat, trouble breathing, nausea and vomiting. Get emergency medical help if you get these symptoms after taking Lunesta.
Call your healthcare professional right away if you have any of the above side effects or any other side effects that worry you while using Lunesta.
The most common side effects of Lunesta are:
- unpleasant taste in mouth, dry mouth
- drowsiness
- dizziness
- headache
- symptoms of the common cold
- You may still feel drowsy the next day after taking Lunesta. Do not drive or do other dangerous activities after taking Lunesta until you feel fully awake.
These are not all the side effects of Lunesta. Ask your healthcare professional or pharmacist for more information. Call your healthcare professional for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Lunesta
- Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
- Do not use Lunesta for a condition for which it was not prescribed.
- Do not share Lunesta with other people, even if you think they have the same symptoms that you have. It may harm them and is against the law.
This Medication Guide summarizes the most important information about Lunesta. If you would like more information, talk with your doctor. You can ask your healthcare professional or pharmacist for information about Lunesta that is written for healthcare professionals.
- For customer service, call 1-888-394-7377.
- To report side effects, call 1-877-737-7226.
- For medical information, call 1-800-739-0565.
How should I store Lunesta?
- Store Lunesta at room temperature, between 59°F to 86°F (15°C to 30°C).
- Do not use Lunesta after the expiration date.
- Keep Lunesta and all medicines out of reach of children.
What are the ingredients in Lunesta?
Active Ingredient: eszopiclone
Inactive Ingredients: calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and triacetin. In addition, both the 1 mg and 3 mg tablets contain FD&C Blue #2.
Label
1 MG BOTTLE LABEL – PRINCIPAL DISPLAY PANEL – 1mg
- NDC 63402-190-30
- 30 Tablets
- Lunesta®
- (eszopiclone) Tablets
- 1 mg C-IV
- DISPENSER: Each time Lunesta is
- dispensed give the patient a medication
- guide, also provided at www.Lunesta.com
- or 1-888-394-7377.
- SUNOVION
- Rx Only

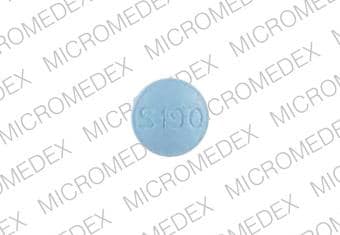
2 MG BOTTLE LABEL – PRINCIPAL DISPLAY PANEL – 2mg
- NDC 63402-191-03
- 30 Tablets
- Lunesta®
- (eszopiclone) Tablets
- 2 mg C-IV
- DISPENSER: Each time Lunesta is
- dispensed give the patient a medication
- guide, also provided at www.Lunesta.com
- or 1-888-394-7377.
- SUNOVION
- Rx Only

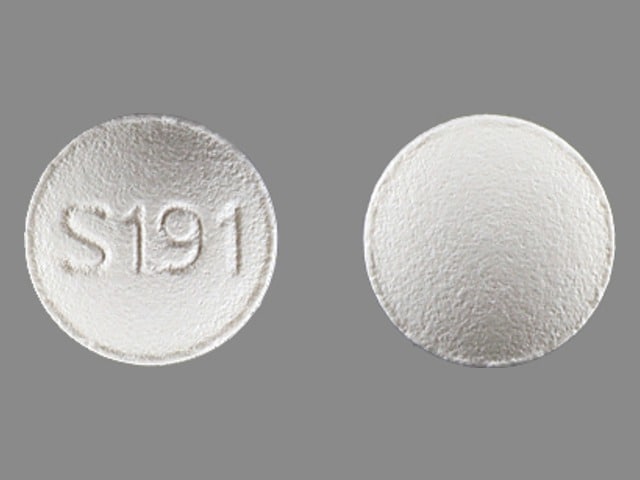
3 MG BOTTLE LABEL – PRINCIPAL DISPLAY PANEL – 3mg
- NDC 63402-193-03
- 30 Tablets
- Lunesta®
- (eszopiclone) Tablets
- 3 mg C-IV
- DISPENSER: Each time Lunesta is
- dispensed give the patient a medication
- guide, also provided at www.Lunesta.com
- or 1-888-394-7377.
- SUNOVION
- Rx Only


SRC: NLM .
