Lumakras
Generic name: sotorasib
Dosage form: tablets
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Lumakras?
Lumakras is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC) that has spread to other parts of the body or cannot be removed by surgery, and whose tumor has an abnormal KRAS G12C gene, and who have received at least one prior treatment for their cancer.
Your healthcare provider will perform a test to make sure that Lumakras is right for you.
It is not known if Lumakras is safe and effective in children.
Description
Sotorasib is an inhibitor of the RAS GTPase family. The molecular formula is C30H30F2N6O3, and the molecular weight is 560.6 g/mol. The chemical name of sotorasib is 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]-4-[(2S)-2- methyl-4-(prop-2-enoyl)piperazin-1-yl]pyrido[2,3-d]pyrimidin-2(1H)-one.
The chemical structure of sotorasib is shown below:
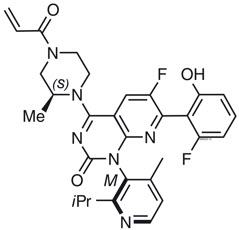
Sotorasib has pKa values of 8.06 and 4.56. The solubility of sotorasib in the aqueous media decreases over the range pH 1.2 to 6.8 from 1.3 mg/mL to 0.03 mg/mL.
LUMAKRAS is supplied as film-coated tablets for oral use containing 120 mg of sotorasib. Inactive ingredients in the tablet core are microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate. The film coating material consists of polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide yellow.
Mechanism of Action
Sotorasib is an inhibitor of KRASG12C, a tumor-restricted, mutant-oncogenic form of the RAS GTPase, KRAS. Sotorasib forms an irreversible, covalent bond with the unique cysteine of KRASG12C, locking the protein in an inactive state that prevents downstream signaling without affecting wild-type KRAS. Sotorasib blocked KRAS signaling, inhibited cell growth, and promoted apoptosis only in KRAS G12C tumor cell lines. Sotorasib inhibited KRASG12C in vitro and in vivo with minimal detectable off-target activity. In mouse tumor xenograft models, sotorasib-treatment led to tumor regressions and prolonged survival, and was associated with anti-tumor immunity in KRAS G12C models.
What should I tell my healthcare provider before taking Lumakras?
Before taking Lumakras, tell your healthcare provider about all your medical conditions, including if you:
- have liver problems.
- have lung or breathing problems other than lung cancer.
- are pregnant or plan to become pregnant. It is not known if Lumakras will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Lumakras passes into your breast milk. Do not breastfeed during treatment with Lumakras and for 1 week after the final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, dietary, and herbal supplements. Lumakras can affect the way some other medicines work and some other medicines can affect the way Lumakras works.
Especially tell your healthcare provider if you take antacid medicines, including Proton Pump Inhibitor (PPI) medicines or H2 blockers during treatment with Lumakras. Ask your healthcare provider if you are not sure.
How should I take Lumakras?
- Take Lumakras exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking Lumakras unless your healthcare provider tells you to.
- Take Lumakras 1 time each day, at about the same time each day.
- Take Lumakras with or without food.
- Swallow Lumakras tablets whole. Do not chew, crush, or split tablets.
- If you cannot swallow Lumakras tablets whole:
- Place your daily dose of Lumakras in a glass of 4 ounces (120 mL) of non-carbonated, room temperature water without crushing the tablets. Do not use any other liquids.
- Stir until the tablets are in small pieces (the tablets will not completely dissolve). The color of the mixture may be pale yellow to bright yellow.
- Drink the Lumakras and water mixture right away or within 2 hours of preparing. Do not chew pieces of the tablet.
- Rinse the glass with an additional 4 ounces (120 mL) of water and drink to make sure that you have taken the full dose of Lumakras.
- If you do not drink the mixture right away, stir the mixture again before drinking.
- If you take an antacid medicine, take Lumakras either 4 hours before or 10 hours after the antacid.
- If you miss a dose of Lumakras, take the dose as soon as you remember. If it has been more than 6 hours, do not take the dose. Take your next dose at your regularly scheduled time the next day. Do not take 2 doses at the same time to make up for a missed dose.
- If you vomit after taking a dose of Lumakras, do not take an extra dose. Take your next dose at your regularly scheduled time the next day.
What are the possible side effects of Lumakras?
Lumakras may cause serious side effects, including:
- Liver problems. Lumakras may cause abnormal liver blood test results. Your healthcare provider should do blood tests before starting and during treatment with Lumakras to check your liver function. Tell your healthcare provider right away if you get any signs or symptoms of liver problems, including:
- Lung or breathing problems. Lumakras may cause inflammation of the lungs that can lead to death. Tell your healthcare provider or get emergency medical help right away if you have new or worsening shortness of breath, cough, or fever.
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Lumakras if you develop side effects.
The most common side effects of Lumakras include:
- diarrhea
- muscle or bone pain
- nausea
- tiredness
- liver problems
- cough
- changes in liver function tests
- changes in certain other blood tests
These are not all the possible side effects of Lumakras.
Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Amgen at 1-800-772-6436 (1-800-77-AMGEN).
General information about the safe and effective use of Lumakras
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Lumakras for a condition for which it was not prescribed. Do not give Lumakras to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Lumakras that is written for healthcare professionals.
How should I store Lumakras?
- Store Lumakras at room temperature between 68°F to 77°F (20°C to 25°C).
- The bottle has a child-resistant closure.
Keep Lumakras and all medicines out of the reach of children.
What are the ingredients in Lumakras?
Active Ingredient: sotorasib
Inactive Ingredients: microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, and magnesium stearate.
Tablet film coating material contains polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide yellow.
Label
PRINCIPAL DISPLAY PANEL – 120 MG TABLET BOTTLE CARTON LABEL
- NDC 55513-488-24
- LUMAKRAS™
(sotorasib) TABLETS - 120 mg
- Each tablet contains 120 mg sotorasib.
- Store at 20°C to 25°C (68°F to 77°F).
Excursions permitted from 15°C to 30°C
(59°F to 86°F). - Recommended Dosage: See Prescribing
Information. - AMGEN®

SRC: NLM .
