Livmarli
Generic name: maralixibat
Dosage form: oral solution
Drug class: Miscellaneous GI agents
Medically reviewed by A Ras MD.
What is Livmarli?
Livmarli is a prescription medicine used to treat cholestatic pruritus (itch) in patients with Alagille syndrome 1 year of age and older. It is not known if Livmarli is safe and effective in children under 1 year of age. It is not known if Livmarli is safe and effective in adults 65 years of age and older.
Description
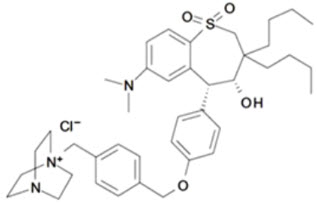
LIVMARLI (maralixibat) oral solution is an ileal bile acid transporter (IBAT) inhibitor. Maralixibat is present as a chloride salt with the chemical name 1-[[4-[[4-[(4R,5R)-3,3-dibutyl-7-(dimethylamino)-2,3,4,5-tetrahydro-4-hydroxy-1,1-dioxido-1-benzothiepin-5-yl]phenoxy]methyl]phenyl]methyl]-4-aza-1-azoniabicyclo[2.2.2]octane chloride. The molecular formula of maralixibat chloride is C40H56ClN3O4S with a molecular weight of 710.42. It has the following chemical structure:
LIVMARLI is supplied in a multiple-dose bottle containing 9.5 mg of maralixibat per mL (equivalent to 10 mg of maralixibat chloride per mL). The oral solution contains the following inactive ingredients: edetate disodium, grape flavor, propylene glycol, purified water, and sucralose. The pH of the oral solution is 3.8 – 4.8.
Mechanism of Action
Maralixibat is a reversible inhibitor of the ileal bile acid transporter (IBAT). It decreases the reabsorption of bile acids (primarily the salt forms) from the terminal ileum.
Pruritus is a common symptom in patients with ALGS and the pathophysiology of pruritus in patients with ALGS is not completely understood. Although the complete mechanism by which maralixibat improves pruritus in ALGS patients is unknown, it may involve inhibition of the IBAT, which results in decreased reuptake of bile salts, as observed by a decrease in serum bile acids
What should I tell my healthcare provider before taking Livmarli?
Before you take Livmarli, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if Livmarli will harm your unborn baby. Tell your healthcare provider right away if you think that you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Livmarli passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you take Livmarli.
Tell your healthcare provider about all medicines that you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. Livmarli may affect the way some other medicines work, and some other medicines may affect the way Livmarli works.
How should I take Livmarli?
Read the “Instructions for Use” that comes with Livmarli for information about the right way to prepare and take Livmarli.
- Before you take Livmarli for the first time, talk to your healthcare provider or pharmacist about how to measure your prescribed dose.
- Take Livmarli exactly as your healthcare provider tells you to.
- Your healthcare provider may start you on a low dose of Livmarli and then increase the dose, especially if you have not taken Livmarli.
- Do not change your dose of Livmarli unless your healthcare provider tells you to.
- Livmarli is taken by mouth, 1 time each day, 30 minutes before your first meal of the day.
- If you miss a dose of Livmarli and it is:
- 12 hours or less from the time you usually take Livmarli, take the missed dose as soon as possible. Then take your next dose at the usual time.
- more than 12 hours from the time you usually take Livmarli, do not take the missed dose. Take your next dose at the usual time.
- If you take too much Livmarli, call your healthcare provider or go to the nearest emergency room right away.
- If you take a medicine that lowers cholesterol by binding bile acids, such as cholestyramine, colesevelam, or colestipol, take it at least 4 hours before or 4 hours after you take Livmarli. Ask your healthcare provider if you are not sure if you take these medicines.
What are the possible side effects of Livmarli?
Livmarli can cause serious side effects, including:
- Changes in liver tests. Changes in certain liver tests are common in patients with Alagille syndrome but may worsen during treatment with Livmarli. These changes may be a sign of liver injury and can be serious. Your healthcare provider should do blood tests before starting and during treatment with Livmarli to check your liver function. Tell your healthcare provider right away if you get any signs or symptoms of liver problems, including:
- Stomach and intestinal (gastrointestinal) problems. Livmarli can cause stomach and intestinal problems, including diarrhea, stomach pain, and vomiting during treatment. Tell your healthcare provider right away if you have any of these symptoms more often or more severely than normal for you.
- A condition called Fat Soluble Vitamin (FSV) Deficiency caused by low levels of certain vitamins (vitamin A, D, E, and K) stored in body fat. FSV deficiency is common in patients with Alagille syndrome but may worsen during treatment with Livmarli. Your healthcare provider should do blood tests before starting and during treatment with Livmarli.
- Other common side effects reported during treatment with Livmarli were bone fractures and gastrointestinal bleeding.
Your healthcare provider may change your dose or temporarily or permanently stop treatment with Livmarli if you have certain side effects.
These are not all of the possible side effects of Livmarli. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Livmarli
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Livmarli for a condition for which it was not prescribed. Do not give Livmarli to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Livmarli that is written for health professionals.
How should I store Livmarli?
- Store Livmarli at room temperature between 68ºF and 77ºF (20ºC and 25ºC).
- Always store Livmarli with the cap on the bottle.
- Throw away any remaining Livmarli, following the steps below, 45 days after first opening the bottle.
- Mix medicine with an unappealing substance such as dirt, cat litter, or used coffee grounds;
- Place the mixture in a container such as a sealed plastic bag;
- Throw away the container in your trash at home; and
- Delete all personal information on the prescription label of the empty medicine bottle, then throw away or recycle the empty bottle.
Keep Livmarli and all medicines out of the reach of children.
What are the ingredients in Livmarli?
Active ingredients: maralixibat chloride
Inactive ingredients: edetate disodium, grape flavor, propylene glycol, purified water, and sucralose.
For more information, go to www.LIVMARLI.com or call 1-855-MRM-4YOU
Instructions for use for Livmarli
Livmarli [liv-MAR-lee]
(maralixibat)
oral solution
This Instructions for Use contains information on how to take Livmarli. Read this Instructions for Use before you start taking Livmarli for the first time and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Follow your healthcare provider’s instructions for the dose of Livmarli to give.
Ask your healthcare provider or pharmacist if you have questions about how to prepare or give the prescribed dose of Livmarli.
Important Information about Measuring Livmarli
- Before you give Livmarli for the first time, talk to your healthcare provider or pharmacist about how to correctly measure your prescribed dose.
- You may be given one or more dosing dispensers of different sizes as shown in Table 1 below. Always use the correct dosing dispenser size provided with Livmarli based on your current prescribed dose.
Table 1
| Dose (mL) | Dosing Dispenser Size (mL) |
| 0.1 to 0.5 | 0.5 |
| 0.6 to 1 | 1 |
| 1.25 to 3 | 3 |
- Your prescribed dose may change over time. Use the table above to choose the correct dosing dispenser size for your prescribed dose.
- If you do not have the correct dosing dispenser size for your prescribed dose, please contact your healthcare provider or pharmacist.
- Dosing dispenser may be used for 30 days if cleaned correctly (see Section B). After 30 days, replace the dosing dispenser with a new one.
- Do not use a household teaspoon or any other dosing device to measure the dose.
- Do not open more than one bottle at a time.
- Do not give more than the prescribed dose.
- Do not use Livmarli after 45 days of first opening the bottle or after the discard date listed on the pharmacy label (see Section C).
- When you start a new bottle of Livmarli, use a new dosing dispenser.
Storage Information
- Store Livmarli at room temperature, between 68°F and 77°F (20°C and 25°C).
- Always store Livmarli with the cap on the bottle.
- Store the dosing dispenser in a clean, dry place when not in use.
- Keep Livmarli and all medicines out of the reach of children.
You Will Receive
Each Package Contains:
Livmarli (9.5 mg/mL):
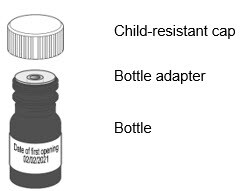
Figure A
Note: If the date of first opening or discard date is not already written on the bottle label by your pharmacist, write the date (bottle open date) on the Livmarli bottle.
Dosing Dispensers, Provided Separately by Your Pharmacist:
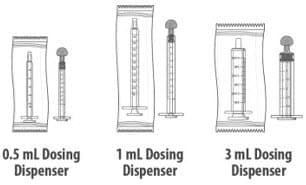
Figure B
Note: Dosing dispenser sizes shown are for example only.
Section A: Prepare and give Livmarli
Step 1: Draw Dose
1.1: Open the bottle by pushing down firmly on the child-resistant cap and turning the cap left (counter-clockwise) (See Figure C). Do not throw away the child-resistant cap.
Figure C
1.2: If using a new dosing dispenser, remove the dosing dispenser from the wrapper (See Figure D). Throw away (dispose of) the wrapper in household trash.
If using a used dosing dispenser, make sure the dosing dispenser has been cleaned (See Section B).
If there is a cap on the dosing dispenser, remove it and throw away (dispose of) the cap into the household trash (See Figure E).
Important:
- Make sure you use the correct dosing dispenser size for your prescribed dose (See TABLE 1).
- After 30 days, replace with a new dosing dispenser provided.
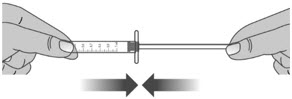
- Check the dosing dispenser for any damage to the barrel, plunger or tip (See Figure F). If you cannot see the dosage marking or if it becomes difficult to move the plunger, replace with a new dosing dispenser.Note: You can mark the prescribed dose on the dosing dispenser with a thin permanent marker to draw up the prescribed dose more easily.
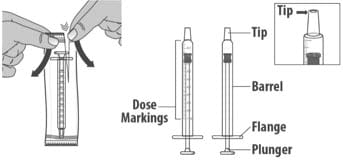
Figure D
Figure E
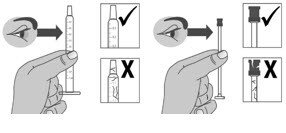
Figure F
1.3: Push the plunger down fully to remove air from the dosing dispenser (See Figure G).
Figure G
1.4: Make sure the cap is removed from bottle and insert the tip of the dosing dispenser into the bottle. The tip of the dosing dispenser should fit securely into the hole of the bottle (See Figure H).
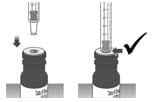
Figure H
1.5: Keep the dosing dispenser in place and turn the bottle upside down (See Figure I).
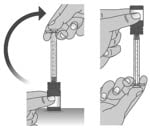
Figure I
1.6: Pull back the plunger slowly until the top of the plunger is even with the marking on the barrel of the dosing dispenser for your prescribed dose of Livmarli (See Figure J).
See Figure K on how to align the plunger with your prescribed dose.
Note: The medicine should appear colorless to light yellow and clear. If it is not, do not use the medicine and contact your pharmacist.
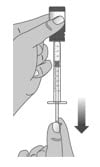
Figure J
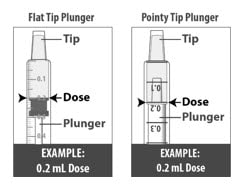
Figure K
Note: Your dose may be different than the dose shown in the Figures.
1.7: Check the dosing dispenser for air bubbles. If you see any air bubbles, fully push the plunger so that the medicine flows back into bottle and withdraw the prescribed dose (See Figure L).
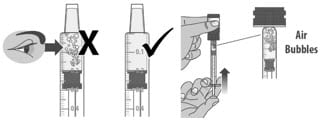
Figure L
1.8: When you have measured the correct dose, leave the dosing dispenser in the bottle, and turn the bottle right side up (See Figure M).
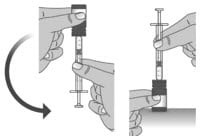
Figure M
1.9: Carefully remove the dosing dispenser from the bottle by pulling straight up on the barrel of the dosing dispenser (See Figure N).
Do not push the dosing dispenser plunger during this step.
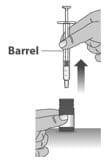
Figure N
Step 2: Give Dose
Note: Livmarli should be taken while sitting up or standing. After taking Livmarli wait a few minutes before lying down.
2.1: Place the tip of the dosing dispenser against the inside of the cheek (See Figure O) and slowly push the plunger all the way in to give the entire dose of Livmarli (See Figure P).
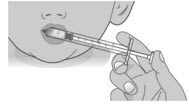
Figure O
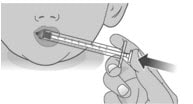
Figure P
2.2: Swallow the dose.
If you are not sure the entire dose was swallowed, do not give another dose. Wait until the next scheduled dose.
2.3: Place the child-resistant cap back on the bottle and turn the cap to the right (clockwise) (See Figure Q).
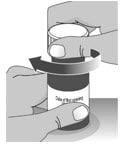
Figure Q
Section B: Cleaning instructions for the dosing dispenser
Step 1: Rinse Dosing Dispenser
1.1: Fill a cup with water (See Figure R).

Figure R
1.2: Clean the dosing dispenser by pulling back on the plunger slowly to fill the dosing dispenser with water from the cup (See Figure S).
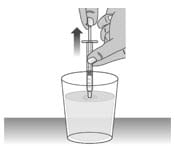
Figure S
1.3: Over a sink, push the water out of the dosing dispenser (See Figure T). Repeat several times to make sure that all of the Livmarli has been removed.
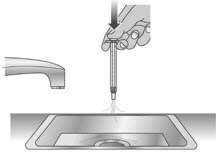
Figure T
Step 2: Dry Dosing Dispenser
2.1: Remove the plunger from the barrel of the dosing dispenser by pulling the plunger and barrel away from each other (See Figure U).
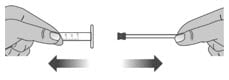
Figure U
2.2: Shake off excess water (See Figure V).
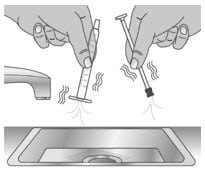
Figure V
2.3: Place the plunger and barrel on a clean, dry paper towel to air dry. Store the dosing dispenser in a clean, dry place until your next dose (See Figure W).
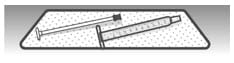
Figure W
Before you give the next dose, put the dosing dispenser back together by pushing the plunger into the barrel (See Figure X).
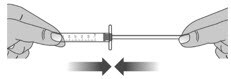
Figure X
Section C: Disposal
- Throw away (dispose of) the bottle of Livmarli following the steps below 45 days after first opened, even if there is still medicine in it.
- Mix medicine with an unappealing substance such as dirt, cat litter, or used coffee grounds;
- Place the mixture in a container such as a sealed plastic bag;
- Throw away the container in your trash at home; and
- Delete all personal information on the prescription label of the empty medicine bottle, then throw away or recycle the empty bottle.
- Throw away (dispose of) used dosing dispensers in the household trash.
Label
PRINCIPAL DISPLAY PANEL – 30 ML BOTTLE LABEL
- NDC 79378-110-01
Rx only - Livmarli™
(maralixibat) oral solution
9.5 mg/mL - Recommended Dosage:
See prescribing information - 30 mL
SRC: NLM .
