Levemir
Generic name: insulin detemir
Brand names: Levemir, Levemir FlexTouch
Drug class: Insulin
Medically reviewed by A Ras MD.
What is Levemir?
Levemir is a man-made insulin that is used to control high blood sugar in adults and children with diabetes mellitus.
Levemir is not meant for use to treat diabetic ketoacidosis.
Description
LEVEMIR (insulin detemir injection) is a solution for subcutaneous use. Insulin detemir is a long-acting recombinant human insulin analog. LEVEMIR is produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae followed by chemical modification.
Insulin detemir differs from human insulin in that the amino acid threonine in position B30 has been omitted, and a C14 fatty acid chain has been attached to the amino acid B29. Insulin detemir has a molecular formula of C267H402O76N64S6 and a molecular weight of 5916.9. It has the following structure:
Figure 1: Structural Formula of Insulin Detemir
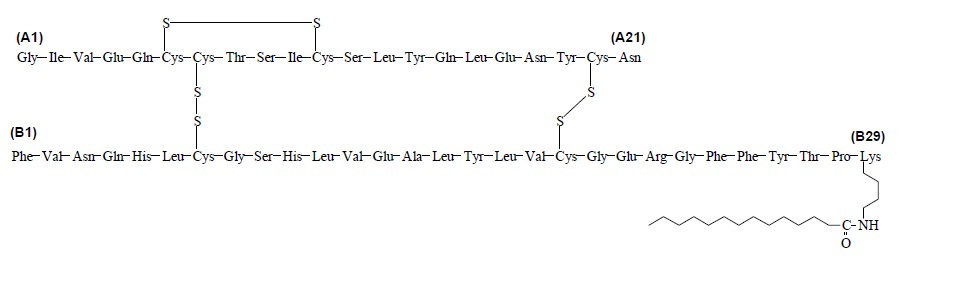
LEVEMIR is a clear, colorless, aqueous, neutral sterile solution. Each milliliter of LEVEMIR contains 100 units (14.2 mg/mL) insulin detemir, 65.4 mcg zinc, 2.06 mg m-cresol, 16.0 mg glycerol, 1.80 mg phenol, 0.89 mg disodium phosphate dihydrate, 1.17 mg sodium chloride, and water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. LEVEMIR has a pH of approximately 7.4.
Mechanism of Action
The primary activity of insulin, including LEVEMIR, is regulation of glucose metabolism. Insulins and its analogs lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin also inhibits lipolysis and proteolysis, and enhances protein synthesis.
What is the most important information I should know about Levemir?
Do not share your Levemir FlexTouch with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
Who should not take Levemir?
Do not take Levemir if you:
- have an allergy to Levemir or any of the ingredients in Levemir.
What should I tell my healthcare provider before taking Levemir?
Before taking Levemir, tell your healthcare provider about all your medical conditions including, if you are:
- pregnant, planning to become pregnant, or are breastfeeding.
- taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
Before you start taking Levemir, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take Levemir?
- Read the Instructions for Use that come with your Levemir.
- Take Levemir exactly as your healthcare provider tells you to.
- Know the type and strength of insulin you take. Do not change the type of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
- Do not reuse or share your needles or syringes with other people. You may give other people a serious infection, or get a serious infection from them.
- Levemir is injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- Never inject Levemir into a vein or muscle.
- Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
What should I avoid while taking Levemir?
While taking Levemir do not:
- Drive or operate heavy machinery, until you know how Levemir affects you.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of Levemir?
Levemir may cause serious side effects that can lead to death, including:
Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- dizziness or light-headedness
- sweating
- confusion
- headache
- blurred vision
- slurred speech
- shakiness
- fast heart beat
- anxiety, irritability, or mood changes
- hunger
Your insulin dose may need to change because of:
- change in level of physical activity or exercise
- weight gain or loss
- increased stress
- illness
- change in diet
Other common side effects of Levemir may include:
- Reactions at the injection site
- itching, rash, serious allergic reactions (whole body reactions)
- skin thickening or pits at the injection site (lipodystrophy)
- weight gain
- swelling of your hands and feet
Get emergency medical help if you have:
- trouble breathing
- shortness of breath
- fast heartbeat
- swelling of your face, tongue, or throat
- sweating
- extreme drowsiness
- dizziness
- confusion
These are not all the possible side effects of Levemir. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Levemir?
- Do not freeze Levemir. Do not use Levemir if it has been frozen.
- Keep Levemir away from heat or light.
- All unopened vials:
- Store unopened Levemir vials in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unopened vials may be used until the expiration date printed on the label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 42 days, if they are stored at room temperature below 86°F (30°C).
- After vials have been opened:
- Opened Levemir vials can be stored in the refrigerator at 36°F to 46°F (2°C to 8°C) or at room temperature below 86°F (30°C).
- Throw away all opened Levemir vials after 42 days, even if they still have insulin left in them.
Keep out of sight and reach of children.
What are the ingredients in Levemir?
Active Ingredient: insulin detemir
Inactive Ingredients: zinc, m-cresol, glycerol, phenol, disodium phosphate dihydrate, sodium chloride and water for injection. Hydrochloric acid or sodium hydroxide may be added.
Label
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 10 ML VIAL
- Levemir®
- Insulin detemir injection
- NDC 0169-3687-12
- List 368712
- 100 units/mL (U-100)
- For subcutaneous use only
- Rx Only
- One 10 mL Vial
- novo nordisk®

PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 3 ML FLEXTOUCH
- NDC 0169-6438-10 List 643810
- Levemir® FlexTouch®
- Insulin detemir injection
- For Single Patient Use Only
- 100 units/mL (U-100)
- 5×3 mL Prefilled Pens
- For subcutaneous use only
- Rx Only
- Recommended for use with
- NovoFine®, NovoFine® Plus or
- NovoTwist® disposable needles.
- Keep in a cold place until first use.
- Store at 2° – 8°C (36° – 46°F).
- Avoid freezing.
- Protect from light.
- Dispense in this sealed carton.

SRC: NLM .
