Koselugo
Generic name: selumetinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Koselugo?
Koselugo is a prescription medicine that is used to treat children 2 years of age and older with neurofibromatosis type 1 (NF1) who have plexiform neurofibromas that cannot be completely removed by surgery.
It is not known if Koselugo is safe and effective in children under 2 years of age.
Description
Selumetinib is a kinase inhibitor. The chemical name is 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-6-[(2-hydroxyethoxy)carbamoyl]-1-methyl-1H-benzimidazol-3-ium hydrogen sulfate. The molecular formula for selumetinib sulfate is C17H17BrClFN4O7S and the relative molecular mass is 555.76 g/mol. Selumetinib sulfate has the following structural formula:
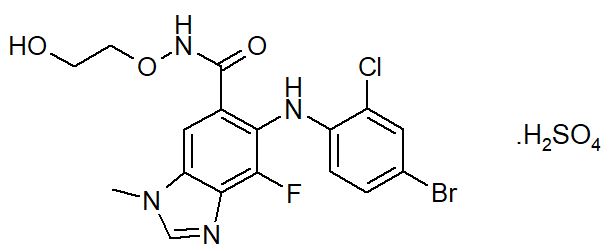
Selumetinib sulfate is a white to yellow monomorphic crystalline powder that exhibits a pH dependent solubility. Selumetinib sulfate is freely soluble at pH < 1.5, sparingly soluble in the pH range at 1.5 to 3 and slightly soluble at pH > 3. Selumetinib sulfate has two ionizable functions with pKa values of 2.8 and 8.4.
KOSELUGO (selumetinib) 10 mg capsules for oral use, contain 10 mg selumetinib (equivalent to 12.1 mg selumetinib sulfate) and the excipient, vitamin E polyethylene glycol succinate. The capsule shell contains hypromellose, carrageenan, potassium chloride, titanium dioxide, carnauba wax, and purified water. The capsule is imprinted with black ink that contains shellac, iron oxide black, propylene glycol and ammonium hydroxide.
KOSELUGO (selumetinib) 25 mg capsules for oral use, contain 25 mg selumetinib (equivalent to 30.25 mg selumetinib sulfate) and the excipient, vitamin E polyethylene glycol succinate. The capsule shell contains hypromellose, carrageenan, potassium chloride, titanium dioxide, FD&C blue 2, ferric oxide yellow, purified water, carnauba wax, and/or corn starch. The capsule is imprinted with black ink that contains ferric oxide red, ferric oxide yellow, FD&C Blue 2 aluminum lake, carnauba wax, shellac, and glyceryl monooleate.
Mechanism of Action
Selumetinib is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). MEK1/2 proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. Both MEK and ERK are critical components of the RAS-regulated RAF-MEK-ERK pathway, which is often activated in different types of cancers.
In genetically modified mouse models of NF1 that generate neurofibromas that recapitulate the genotype and phenotype of human NF1, oral dosing of selumetinib inhibited ERK phosphorylation, and reduced neurofibroma numbers, volume, and proliferation.
What should I tell my healthcare provider before taking Koselugo?
Before taking Koselugo, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have eye problems.
- are pregnant or plan to become pregnant. Koselugo can harm your unborn baby.
- Your healthcare provider should check to see if you are pregnant before you begin treatment with Koselugo.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with Koselugo and for 1 week after your last dose.
- Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment with Koselugo and for 1 week after your last dose.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Koselugo.
- are breastfeeding or plan to breastfeed. It is not known if Koselugo passes into your breast milk.
- Do not breastfeed during treatment with Koselugo and for 1 week after your last dose.
- Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements. Especially tell your healthcare provider if you are taking aspirin, blood thinners, or other medicines to treat blood clots. Koselugo contains vitamin E which may increase your risk of bleeding.
How should I take Koselugo?
- Take Koselugo exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking Koselugo unless your healthcare provider tells you to.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Koselugo if you have side effects.
- Your healthcare provider will decide on the right dose of Koselugo based on your weight or size (body surface area) and how many capsules of Koselugo to take.
- Koselugo should be taken around the same time each day, about 12 hours apart.
- Take Koselugo on an empty stomach. Do not eat food for 2 hours before your dose and 1 hour after your dose.
- Swallow Koselugo capsules whole with water. Do not chew, dissolve, or open the capsules.
- If you miss a dose of Koselugo, take it as soon as you remember. If it is less than 6 hours before your next scheduled dose, take your next dose at your regular time. Do not make up for the missed dose.
- If you vomit at any time after taking Koselugo, do not take an additional dose. Take your next dose at your regular time.
What should I avoid while taking Koselugo?
Do not drink grapefruit juice, eat grapefruit or take supplements that contain grapefruit or St. John’s Wort during treatment with Koselugo.
What are the possible side effects of Koselugo?
Koselugo may cause serious side effects, including:
- Heart Problems. Koselugo can lower the amount of blood pumped by your heart which is common and can also be severe. Your healthcare provider will do tests before and during treatment with Koselugo to check how well your heart is working. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- persistent coughing or wheezing
- shortness of breath
- swelling of your ankles and feet
- tiredness
- increased heart rate
- Eye Problems. Koselugo can cause eye problems that can lead to blindness. Your healthcare provider will check your vision before and during treatment with Koselugo. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- blurred vision
- loss of vision
- dark spots in your vision (floaters)
- other changes to your vision
- Severe diarrhea. Diarrhea is common with Koselugo and can also be severe. Tell your healthcare provider right away the first time that you get diarrhea during treatment with Koselugo. Your healthcare provider may give you medicine to help control your diarrhea and may tell you to drink more fluids.
- Skin Rash. Skin rashes are common with Koselugo and can also be severe. Tell your healthcare provider if you get any of the following signs or symptoms:
- rash that covers a large area of your body
- peeling skin
- blisters
- Muscle problems (rhabdomyolysis). Muscle problems are common with Koselugo and can also be severe. Treatment with Koselugo may increase the level of enzyme in your blood called creatine phosphokinase (CPK) and may be a sign of muscle damage. Your healthcare provider should do a blood test to check your blood levels of CPK before you start taking Koselugo and during treatment. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- muscle aches or pain
- muscle spasms and weakness
- dark, reddish urine
Your healthcare provider may change your dose, temporarily stop, or permanently ask you to stop taking Koselugo if you have any of these side effects.
The most common side effects of Koselugo are:
- vomiting
- stomach pain
- nausea
- dry skin
- feeling of tiredness, weakness or lacking energy
- muscle and bone pain
- fever
- inflammation of the mouth
- headache
- redness around the fingernails
- itching
These are not all the possible side effects of Koselugo.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Koselugo
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Koselugo for a condition for which it was not prescribed. Do not give Koselugo to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Koselugo that is written for a healthcare professional.
How should I store Koselugo?
- Store Koselugo at room temperature, between 68°F to 77°F (20°C to 25°C).
- The bottle of Koselugo contains a desiccant packet to reduce moisture. Do not throw away desiccant packet.
- Keep Koselugo in its original bottle.
Keep Koselugo and all medicines out of the reach of children.
What are the ingredients in Koselugo?
Active ingredient: selumetinib.
Inactive ingredients:
Capsule contains: vitamin E polyethylene glycol succinate.
The 10 mg capsule shell contains: hypromellose, carrageenan, potassium chloride, titanium dioxide, carnauba wax, and purified water.
The 10 mg capsule printing ink contains: shellac, iron oxide black, propylene glycol, and ammonium hydroxide.
The 25 mg capsule shell contains: hypromellose, carrageenan, potassium chloride, titanium dioxide, FD&C blue 2, ferric oxide yellow, purified water, carnauba wax and/or corn starch.
The 25 mg printing ink contains: ferric oxide red, ferric oxide yellow, FD&C Blue 2 aluminum lake, carnauba wax, shellac, glyceryl monooleate.
Label
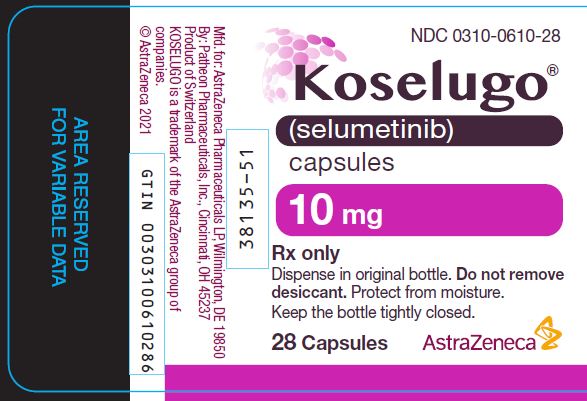
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 10MG
- NDC 0310-0610-28
- Koselugo®
- (selumetinib)
- capsules
- 10 mg
- Rx only
- Dispense in original bottle. Do not remove
- desiccant. Protect from moisture.
- Keep the bottle tightly closed.
- 28 Capsules AstraZeneca
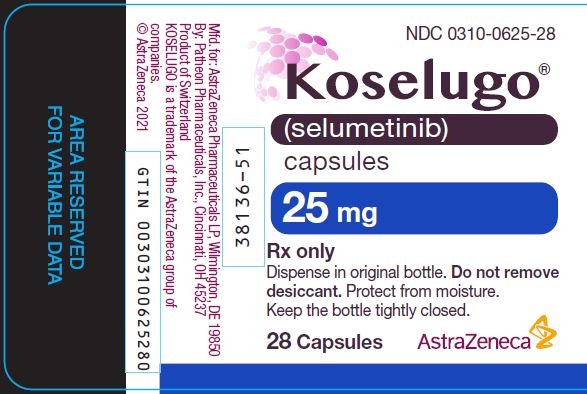
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL 25MG
- NDC 0310-0625-28
- Koselugo®
- (selumetinib)
- capsules
- 25 mg
- Rx only
- Dispense in original bottle. Do not remove
- desiccant. Protect from moisture.
- Keep the bottle tightly closed.
- 28 Capsules AstraZeneca
SRC: NLM .
