Jakafi
Generic name: ruxolitinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Jakafi?
Jakafi is a prescription medicine used to treat adults with certain types of myelofibrosis (MF). adults with polycythemia vera (PV) who have already taken a medicine called hydroxyurea and it did not work well enough or they could not tolerate it, adults and children, 12 years of age and older with acute graft versus host disease (GVHD) who have taken corticosteroids and they did not work well enough.
It is not known if Jakafi is safe or effective in children for treatment of myelofibrosis or polycythemia vera.
Description
Ruxolitinib phosphate is a kinase inhibitor with the chemical name (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile phosphate and a molecular weight of 404.36. Ruxolitinib phosphate has the following structural formula:
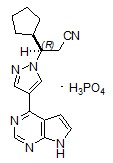
Ruxolitinib phosphate is a white to off-white to light pink powder and is soluble in aqueous buffers across a pH range of 1 to 8.
Jakafi (ruxolitinib) Tablets are for oral administration. Each tablet contains 6.6 mg, 13.2 mg, 19.8 mg, 26.4 mg, or 33 mg of ruxolitinib phosphate equivalent to 5 mg, 10 mg, 15 mg, 20 mg, or 25 mg of ruxolitinib free base, respectively, together with microcrystalline cellulose, lactose monohydrate, magnesium stearate, colloidal silicon dioxide, sodium starch glycolate, povidone and hydroxypropyl cellulose.
Mechanism of Action
Ruxolitinib, a kinase inhibitor, inhibits Janus Associated Kinases (JAKs) JAK1 and JAK2 which mediate the signaling of a number of cytokines and growth factors that are important for hematopoiesis and immune function. JAK signaling involves recruitment of STATs (signal transducers and activators of transcription) to cytokine receptors, activation and subsequent localization of STATs to the nucleus leading to modulation of gene expression.
MF and PV are myeloproliferative neoplasms (MPN) known to be associated with dysregulated JAK1 and JAK2 signaling. In a mouse model of JAK2V617F-positive MPN, oral administration of ruxolitinib prevented splenomegaly, preferentially decreased JAK2V617F mutant cells in the spleen and decreased circulating inflammatory cytokines (e.g., TNF-α, IL-6).
JAK-STAT signaling pathways play a role in regulating the development, proliferation, and activation of several immune cell types important for GVHD pathogenesis. In a mouse model of aGVHD, oral administration of ruxolitinib was associated with decreased expression of inflammatory cytokines in colon homogenates and reduced immune-cell infiltration in the colon.
What should I tell my healthcare provider before taking Jakafi?
Before taking Jakafi, tell your healthcare provider about of your medical conditions, including if you:
- have an infection
- have or had tuberculosis (TB), or have been in close contact with someone who has TB
- have or had hepatitis B
- have or have had liver problems
- have or have had kidney problems or are on dialysis. If you are on dialysis, Jakafi should be taken after your dialysis
- have high level of fat in your blood (high blood cholesterol or triglycerides)
- have had skin cancer in the past
- are pregnant or plan to become pregnant. It is not known if Jakafi will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Jakafi passes into your breast milk. Do not breastfeed during treatment with Jakafi and for 2 weeks after the final dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Taking Jakafi with certain other medicines may affect how Jakafi works. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Jakafi?
- Take Jakafi exactly as your healthcare provider tells you.
- Do not change your dose or stop taking Jakafi without first talking to your healthcare provider.
- You can take Jakafi with or without food.
- Jakafi may also be given through certain nasogastric tubes.
- Tell your healthcare provider if you cannot take Jakafi by mouth. Your healthcare provider will decide if you can take Jakafi through a nasogastric tube.
- Ask your healthcare provider to give you specific instruction on how to properly take Jakafi through a nasogastric tube.
- If you miss a dose of Jakafi, take your next dose at your regular time. Do not take 2 doses at the same time.
- If you take too much Jakafi call your healthcare provider or go to the nearest hospital emergency room right away.
- You will have regular blood tests during your treatment with Jakafi. Your healthcare provider may change your dose of Jakafi or stop your treatment based on the results of your blood tests.
What are the possible side effects of Jakafi?
Jakafi can cause serious side effects including:
Low blood cell counts. Jakafi may cause low platelet counts (thrombocytopenia), low red blood cell counts (anemia), and low white blood cell counts (neutropenia). If you develop bleeding, stop Jakafi and call your healthcare provider. Your healthcare provider will do a blood test to check your blood cell counts before you start Jakafi and regularly during your treatment with Jakafi. Tell your healthcare provider right away if you develop or have worsening of any of these symptoms:
- unusual bleeding
- bruising
- tiredness
- shortness of breath
- fever
Infection. You may be at risk for developing a serious infection during treatment with Jakafi. Tell your healthcare provider if you develop any of the following symptoms of infection:
Skin cancers. Some people who take Jakafi have developed certain types of non-melanoma skin cancers. Tell your healthcare provider if you develop any new or changing skin lesions during treatment with Jakafi.
Cholesterol increases. You may have changes in your blood cholesterol levels. Your healthcare provider will do blood tests to check your cholesterol levels during treatment with Jakafi.
The most common side effects of Jakafi in adults with certain types of MF and PV include:
- low platelet counts (thrombocytopenia)
- low red blood cell counts (anemia)
- bruising
- dizziness
- headache
- diarrhea
The most common side effects of Jakafi in people with acute graft versus host disease (GVHD) include:
- low red blood cell counts (anemia)
- low platelet counts (thrombocytopenia)
- low white blood cell counts (neutropenia)
- infections
- fluid retention
These are not all the possible side effects of Jakafi.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Incyte Corporation at 1-855-463-3463.
General information about the safe and effective use of Jakafi
Medicines are sometimes prescribed for purposes other than those listed in Patient Information. Do not use Jakafi for a condition for which it is not prescribed. Do not give Jakafi to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information that is written for healthcare professionals.
How should I store Jakafi?
- Store Jakafi at room temperature 68°F to 77°F (20°C to 25°C).
Keep Jakafi and all medicines out of the reach of children.
What are the ingredients in Jakafi?
Active ingredient: ruxolitinib phosphate
Inactive ingredients: microcrystalline cellulose, lactose monohydrate, magnesium stearate, colloidal silicon dioxide, sodium starch glycolate, povidone and hydroxypropyl cellulose
Label
5 MG TABLET BOTTLE LABEL
- Rx only
- NDC 50881-005-60
- Jakafi® (Ruxolitinib) Tablets
- 5 mg
- 60 tablets
- Each tablet contains 6.6 mg ruxolitinib phosphate equivalent to 5 mg ruxolitinib free base.


10 MG TABLET BOTTLE LABEL
- Rx only
- NDC 50881-010-60
- Jakafi® (Ruxolitinib) Tablets
- 10 mg
- 60 tablets
- Each tablet contains 13.2 mg ruxolitinib phosphate equivalent to 10 mg ruxolitinib free base.

15 MG TABLET BOTTLE LABEL
- Rx only
- NDC 50881-015-60
- Jakafi® (Ruxolitinib) Tablets
- 15 mg
- 60 tablets
- Each tablet contains 19.8 mg ruxolitinib phosphate equivalent to 15 mg ruxolitinib free base.

20 MG TABLET BOTTLE LABEL
- Rx only
- NDC 50881-020-60
- Jakafi® (Ruxolitinib) Tablets
- 20 mg
- 60 tablets
- Each tablet contains 26.4 mg ruxolitinib phosphate equivalent to 20 mg ruxolitinib free base.


25 MG TABLET BOTTLE LABEL
- Rx only
- NDC 50881-025-60
- Jakafi® (Ruxolitinib) Tablets
- 25 mg
- 60 tablets
- Each tablet contains 33 mg ruxolitinib phosphate equivalent to 25 mg ruxolitinib free base.

SRC: NLM .