Ixempra
Generic name: ixabepilone
Drug class: Mitotic inhibitors
Medically reviewed by A Ras MD.
What is Ixempra?
Ixempra is a cancer medicine. Ixempra is used alone or with another cancer medicine called capecitabine. Ixempra is used to treat breast cancer, when certain other medicines have not worked or no longer work.
Description
IXEMPRA (ixabepilone) is a microtubule inhibitor belonging to a class of antineoplastic agents, the epothilones and their analogs. The epothilones are isolated from the myxobacterium Sorangium cellulosum. Ixabepilone is a semisynthetic analog of epothilone B, a 16-membered polyketide macrolide, with a chemically modified lactam substitution for the naturally existing lactone.
The chemical name for ixabepilone is (1 S,3 S,7 S,10 R,11 S,12 S,16 R)-7,11 -dihydroxy-8,8,10,12,16-pentamethyl-3- [(1 E)-1 -methyl-2-(2-methyl-4-thiazolyl)ethenyl]-17-oxa-4-azabicyclo[14.1.0] heptadecane-5,9-dione, and it has a molecular weight of 506.7. Ixabepilone has the following structural formula:
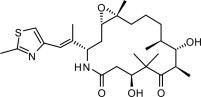
IXEMPRA (ixabepilone) for injection is intended for intravenous infusion only after constitution with the supplied DILUENT and after further dilution with a specified infusion fluid [see dosage ( 2.)]. IXEMPRA (ixabepilone) for injection is supplied as a sterile, non-pyrogenic, single-dose vial providing 15 mg or 45 mg ixabepilone as a lyophilized white powder. The DILUENT for IXEMPRA is a sterile, non-pyrogenic, solution of 52.8% (w/v) purified polyoxyethylated castor oil and 39.8% (w/v) dehydrated alcohol, USP. The IXEMPRA (ixabepilone) for injection and the DILUENT for IXEMPRA are copackaged and supplied as IXEMPRA Kit
Mechanism of Action
Ixabepilone is a semi-synthetic analog of epothilone B. Ixabepilone binds directly to β-tubulin subunits on microtubules, leading to suppression of microtubule dynamics. Ixabepilone suppresses the dynamic instability of αβ-II and αβ-III microtubules. Ixabepilone possesses low in vitro susceptibility to multiple tumor resistance mechanisms including efflux transporters, such as MRP-1 and P-glycoprotein (P-gp). Ixabepilone blocks cells in the mitotic phase of the cell division cycle, leading to cell death.
What is the most important information I should know about Ixempra?
Your healthcare provider should do blood tests to check your liver function:
- before you begin receiving Ixempra
- as needed while you are receiving Ixempra
If blood tests show that you have liver problems, do not receive injections of Ixempra along with the medicine capecitabine. Taking these two medicines together if you have liver problems increases your chance of serious problems. These include: serious infection and death due to a very low white blood cell count (neutropenia).
Who should not use Ixempra?
Do not receive injections of Ixempra if you:
- are allergic to a medicine, such as Taxol, that contains Cremophor EL or polyoxyethylated castor oil.
- have low white blood cell or platelet counts. Your healthcare provider will check your blood counts.
- are also taking a cancer medicine called capecitabine and you have liver problems. See “What is the most important information I should know about Ixempra?”.
What should I tell my healthcare provider before using Ixempra?
Ixempra may not be right for you.
Before you receive Ixempra, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems
- have heart problems or a history of heart problems
- have had an allergic reaction to Ixempra. You will receive medicines before each injection of Ixempra to decrease the chance of an allergic reaction. See “How should I use Ixempra?”
- are pregnant or plan to become pregnant. You should not receive Ixempra during pregnancy because it may harm your unborn baby. Talk with your healthcare provider about how to prevent pregnancy while receiving Ixempra. Tell your healthcare provider right away if you become pregnant or think you are pregnant while receiving Ixempra.
- are breast-feeding. It is not known if Ixempra passes into breast milk. You and your healthcare provider should decide if you will receive Ixempra or breast-feed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Ixempra and certain other medicines may affect each other causing side effects. Ixempra may affect the way other medicines work, and other medicines may affect how Ixempra works. Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider.
How should I use Ixempra?
Ixempra is given by an injection directly into your vein (intravenous infusion). Ixempra is usually given once every three weeks. Each treatment with Ixempra will take about 3 hours.
Your healthcare provider will decide how much Ixempra you will receive and how often you will receive it.
To lower the chance of allergic reaction, you will receive other medicines about 1 hour before each treatment with Ixempra. See “What are the possible side effects of Ixempra?”
If you have an allergic reaction to Ixempra, you will receive a steroid medicine before future doses of Ixempra. You may also need to receive your doses of Ixempra more slowly.
What should I avoid while taking Ixempra?
Ixempra contains alcohol. If you are dizzy or drowsy, avoid activities that may be dangerous, such as driving or operating machinery.
Do not drink grapefruit juice while receiving Ixempra. Drinking grapefruit juice may cause you to have too much Ixempra in your blood and lead to side effects.
What are the possible side effects of Ixempra?
Ixempra may cause serious side effects including:
- Numbness, tingling, or burning in the hands or feet can occur while receiving Ixempra (neuropathy). These symptoms may be new or get worse while you are receiving Ixempra. These symptoms often occur early during treatment with Ixempra. Tell your healthcare provider if you have any of these symptoms. Your dose of Ixempra may need to be decreased, stopped until your symptoms get better, or totally stopped.
- Low white blood cell count (neutropenia). White blood cells help protect the body from infections caused by bacteria. If you get a fever or infection when your white blood cells are very low, you can become seriously ill and die. You may need treatment in the hospital with antibiotic medicines. Your healthcare provider will monitor your white blood cell count often with blood tests. Tell your healthcare provider right away or go to the nearest hospital emergency room if you have a fever (temperature above 100.5° F) or other sign of infection, such as chills, cough, burning or pain when you urinate, any time between treatments with Ixempra.
- Allergic Reactions. Severe allergic reactions can occur with Ixempra and may cause death in rare cases. Allergic reactions are most likely to occur while Ixempra is being injected into your vein. Tell your healthcare provider right away if you get any of the following signs and symptoms of an allergic reaction:
- itching, hives (raised itchy welts), rash
- flushed face
- sudden swelling of face, throat or tongue
- chest tightness, trouble breathing
- feel dizzy or faint
- feel your heart beating (palpitations)
- Harm to an unborn child. See “What should I tell my healthcare provider before taking Ixempra?”
- Heart problems. Ixempra might cause decreased blood flow to the heart, problems with heart function, and abnormal heart beat. This is seen more often in patients who also take capecitabine. Tell your healthcare provider right away if you have any of the following symptoms:
- chest pain,
- difficulty breathing,
- feel your heart beating (palpitations), or
- unusual weight gain.
The most common side effects with Ixempra (ixabepilone) used alone or with capecitabine may include:
- tiredness
- loss of appetite
- disorders of toenails and fingernails
- hair loss
- fever
- decreased red blood cells (anemia)
- joint and muscle pain
- headache
- decreased platelets (thrombocytopenia)
- nausea, vomiting, diarrhea, constipation, and abdominal pain
- sores on the lip, in the mouth and esophagus
- tender, red palms and soles of feet (hand-foot syndrome) that looks like a sunburn; the skin may become dry and peel. There may also be numbness and tingling.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all of the side effects of Ixempra. Ask your healthcare provider or pharmacist for more information if you have questions or concerns.
General information about the safe and effective use of Ixempra
This patient information leaflet summarizes the most important information about Ixempra. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. If you would like more information about Ixempra, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Ixempra that is written for health professionals. For more information about Ixempra, call 1-844-586-8953.
How should I store Ixempra?
Ixempra must be stored in a refrigerator at 2° C to 8° C (36° F to 46° F). Retain in original package until time of use to protect from light.
What are the ingredients in Ixempra?
Active ingredient: ixabepilone
Inactive ingredients: polyoxyl 35 castor oil, alcohol
Label
Principal Display Panel – Carton Label
- Single dose vials NDC 70020-1910-1
- IXEMPRA®Kit
- (ixabepilone) for injection
- 15 mg Rx only
- For intravenous infusion only
- Each carton contains
- 1 vial IXEMPRA (ixabepilone)for injection 15 mg
- 1 vial DILUENT for IXEMPRA 8 mL
- Reconstitution and dilution required(see insert).
- Refrigerate
- Distribute by
- R-Pharm US LLC
- Princeton, NJ 08543 USA

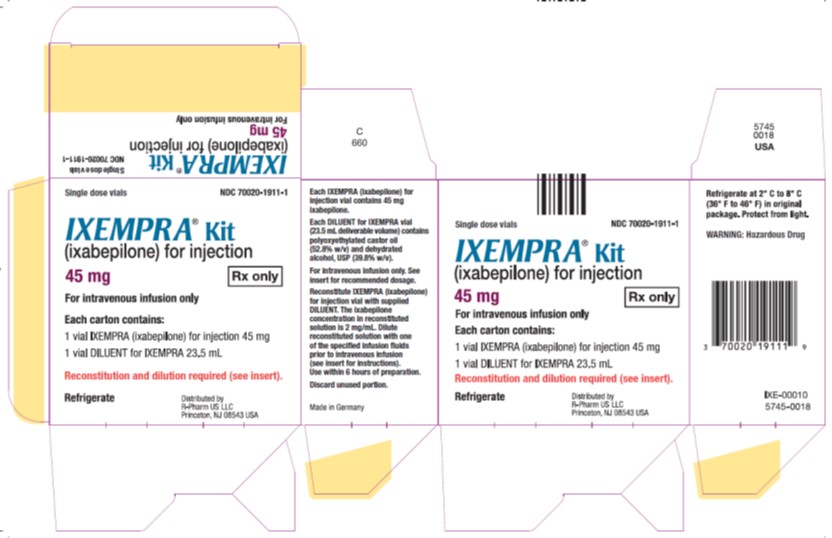
Principal Display Panel – Carton Label
- Single dose vials NDC 70020-1911-1
- IXEMPRA®Kit
- (ixabepilone) for injection
- 45 mg Rx only
- For intravenous infusion only
- Each carton contains
- 1 vial IXEMPRA (ixabepilone)for injection 15 mg
- 1 vial DILUENT for IXEMPRA 23.5 mL
- Reconstitution and dilution required (see insert).
- Refrigerate
- Distribute by
- R-Pharm US LLC
- Princeton, NJ 08543 USA
SRC: NLM .
