Istodax
Generic name: romidepsin
Drug class: Histone deacetylase inhibitors
Medically reviewed by A Ras MD.
What is Istodax?
Description
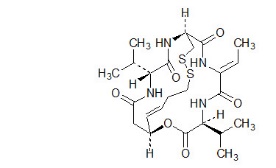
Romidepsin, a histone deacetylase (HDAC) inhibitor, is a bicyclic depsipeptide. At room temperature, romidepsin is a white powder and is described chemically as (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone. The empirical formula is C24H36N4O6S2.
The molecular weight is 540.71 and the structural formula is:
ISTODAX (romidepsin) for injection is intended for intravenous infusion only after reconstitution with the supplied diluent and after further dilution with 0.9% Sodium Chloride, USP.
ISTODAX is supplied as a kit containing 2 vials.
ISTODAX (romidepsin) for injection is a sterile lyophilized white powder and is supplied in a 10 mg single-dose vial containing 11 mg romidepsin, 22 mg povidone, USP, and hydrochloric acid, NF, as a pH adjuster.
Diluent for ISTODAX is a sterile clear solution and is supplied in a single-dose vial containing 2.4 mL (2.2 mL deliverable volume). Diluent for ISTODAX contains 80% (v/v) propylene glycol, USP and 20% (v/v) dehydrated alcohol, USP.
Mechanism of Action
Romidepsin is a histone deacetylase (HDAC) inhibitor. HDACs catalyze the removal of acetyl groups from acetylated lysine residues in histones, resulting in the modulation of gene expression. HDACs also deacetylate non-histone proteins, such as transcription factors. In vitro, romidepsin causes the accumulation of acetylated histones, and induces cell cycle arrest and apoptosis of some cancer cell lines with IC50 values in the nanomolar range. The mechanism of the antineoplastic effect of romidepsin observed in nonclinical and clinical studies has not been fully characterized.
What should I tell my healthcare provider before using Istodax?
Before receiving Istodax, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including an irregular or fast heartbeat, or a condition called QT prolongation.
- have kidney problems
- have liver problems, including a history of hepatitis B
- have problems with the amount of potassium or magnesium in your blood
- have nausea, vomiting, or diarrhea
- are pregnant or plan to become pregnant. Istodax may harm your unborn baby.
- Females who are able to become pregnant:
- Your healthcare provider will perform a pregnancy test before you start treatment with Istodax.
- You should avoid becoming pregnant during treatment with Istodax and for at least 1 month after the last dose.
- You should use effective birth control (contraception) during treatment with Istodax and for at least 1 month after your last dose.
- Istodax may affect the way estrogen-containing birth control works. Talk to your healthcare provider for more information about other types of birth control to use during treatment with Istodax.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Istodax.
- Males with a female sexual partner who can become pregnant:
- Istodax can harm the unborn baby of your partner.
- You should use a condom and avoid fathering a child during treatment with Istodax and for at least one month after treatment with Istodax. Talk to your healthcare provider if this is a concern for you.
- Istodax may cause fertility problems in males and females. Talk to your healthcare provider if this is a concern for you.
- Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if Istodax passes into your breast milk. You and your healthcare provider should decide if you will receive Istodax or breastfeed. Talk to your healthcare provider about the best way to feed your baby while you are being treated with Istodax.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may affect how Istodax works, or Istodax may affect how other medicines work. Especially tell your healthcare provider if you take or use:
- warfarin sodium (Coumadin, Jantoven) or any other blood thinner medicine. Ask your healthcare provider if you are not sure if you are taking a blood thinner. Your healthcare provider may want to test your blood more often.
- a medicine to treat abnormal heartbeats
- St. John’s wort (Hypericum perforatum)
- Dexamethasone (a steroid)
- Medicine for:
- tuberculosis (TB)
- seizures (epilepsy)
- bacterial infections (antibiotics)
- fungal infections (antifungals)
- HIV (AIDS)
- depression
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use Istodax?
- Istodax will be given to you by your healthcare provider or nurse as an intravenous (IV) injection into your vein usually over 4 hours.
- Istodax is usually given on Day 1, Day 8, and Day 15 of a 28-day cycle of treatment.
- Your healthcare provider will decide how long you will receive treatment with Istodax.
- Your healthcare provider will check your blood cell counts and other blood tests regularly during your treatment with Istodax to check for side effects of Istodax. Your healthcare provider may decide to do other tests to check your health as needed.
- Your healthcare provider may stop your treatment, change when you get your treatment, or change the dose of your treatment if you have certain side effects while receiving Istodax.
What are the possible side effects of Istodax?
Istodax may cause serious side effects, including:
- Low blood cell counts: Your healthcare provider will regularly do blood tests to check your blood counts.
- Low platelets: can cause unusual bleeding or bruising under the skin. Talk to your healthcare provider right away if this happens.
- Low red blood cells: may make you feel tired and you may get tired easily. You may look pale and feel short of breath. Tell your healthcare provider if you have these symptoms.
- Low white blood cells: can cause you to get infections, which may be serious.
- Serious infections. People receiving Istodax can develop serious infections that can sometimes lead to death. These infections can happen during treatment and within 30 days after treatment with Istodax. Your risk of infection may be higher if you have had chemotherapy in the past. Tell your healthcare provider right away if you have any of these symptoms of infection:
- Fever
- cough
- shortness of breath with or without chest pain
- burning with urination
- flu-like symptoms
- muscle aches
- worsening skin problems
- Changes in your heartbeat. Your healthcare provider may check your heart by doing an ECG (electrocardiogram) and blood tests to check your potassium and magnesium levels, before you start Istodax treatment. Tell your healthcare provider if you feel an abnormal heartbeat, feel dizzy or faint, have chest pain or shortness of breath.
- Tumor Lysis Syndrome (TLS). TLS is a problem of the rapid breakdown of cancer cells that can happen during your treatment with Istodax. You should drink plenty of fluids in the 3 days after you receive treatment with Istodax. Your healthcare provider may do blood tests to check for TLS and may give you medicine to prevent or treat TLS.
The most common side effects of Istodax include:
- nausea, tiredness, vomiting, diarrhea, and loss of appetite
These are not all the possible side effects of Istodax. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Istodax
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet.
This Patient Information leaflet summarizes the most important information about Istodax. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Istodax that is written for health professionals.
How should I store Istodax?
Istodax (romidepsin) for injection is supplied as a kit containing 2 vials in a single carton. The carton must be stored at 20° to 25°C, excursions permitted between 15° to 30°C. (See USP Controlled Room Temperature.)
What are the ingredients in Istodax?
Active ingredient: romidepsin
Inactive ingredients: povidone. The diluent contains 80% propylene glycol and 20% dehydrated alcohol.
Label
PRINCIPAL DISPLAY PANEL – 10 MG VIAL LABEL
- NDC 59572-962-10
- ISTODAX®
(romidepsin) for injection - Rx Only
- 10 mg/vial
- FOR INTRAVENOUS USE ONLY.
- Directions For Use: See accompanying package
insert for complete reconstitution directions.
Product MUST be reconstituted with 2.2 mL
of supplied Diluent for a concentration of
5 mg/mL and then further diluted in 500 mL
of 0.9% sodium chloride injection, USP.

SRC: NLM .
