Isentress
Generic name: raltegravir
Brand names: Isentress, Isentress HD
Drug class: Integrase strand transfer inhibitor
Medically reviewed by A Ras MD.
What is Isentress and Isentress HD?
Isentress is a prescription HIV medicine used with other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) infection in adults, and in children weighing at least 4.4 pounds (2 kg). HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
Isentress HD is a prescription HIV medicine used with other antiretroviral medicines to treat HIV-1 infection in adults, and in children weighing at least 88 pounds (40 kg).
Isentress should not be used in children who weigh less than 4.4 pounds (2 kg).
Description
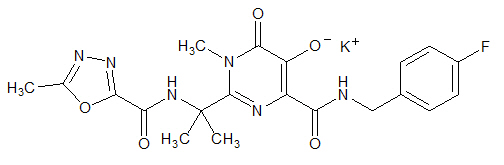
ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl) methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide monopotassium salt.
The empirical formula is C20H20FKN6O5 and the molecular weight is 482.51. The structural formula is:
[Chemical Structure]
Raltegravir potassium is a white to off-white powder. It is soluble in water, slightly soluble in methanol, very slightly soluble in ethanol and acetonitrile and insoluble in isopropanol.
Each 400 mg film-coated tablet of ISENTRESS for oral administration contains 434.4 mg of raltegravir (as potassium salt), equivalent to 400 mg of raltegravir free phenol and the following inactive ingredients: calcium phosphate dibasic anhydrous, hypromellose 2208, lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate. In addition, the film coating contains the following inactive ingredients: black iron oxide, polyethylene glycol 3350, polyvinyl alcohol, red iron oxide, talc and titanium dioxide.
Each 600 mg film-coated tablet of ISENTRESS HD for oral administration contains 651.6 mg of raltegravir (as potassium salt), equivalent to 600 mg of raltegravir free phenol and the following inactive ingredients: croscarmellose sodium, hypromellose 2910, magnesium stearate, microcrystalline cellulose. The film coating contains the following inactive ingredients: ferrosoferric oxide, hypromellose 2910, iron oxide yellow, lactose monohydrate, triacetin and titanium dioxide. The tablet may also contain trace amount of carnauba wax.
Each 100 mg chewable tablet of ISENTRESS for oral administration contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, red iron oxide, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each 25 mg chewable tablet of ISENTRESS for oral administration contains 27.16 mg of raltegravir (as potassium salt), equivalent to 25 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide.
Each packet of ISENTRESS for oral suspension 100 mg, contains 108.6 mg of raltegravir (as potassium salt), equivalent to 100 mg of raltegravir free phenol and the following inactive ingredients: ammonium hydroxide, banana with other natural flavors, carboxymethylcellulose sodium, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, macrogol/PEG 400, magnesium stearate, maltodextrin, mannitol, medium chain triglycerides, microcrystalline cellulose, monoammonium glycyrrhizinate, oleic acid, sorbitol, sucralose and sucrose.
What should I tell my healthcare provider before taking Isentress or Isentress HD?
Before you take Isentress or Isentress HD, tell your doctor about all of your medical conditions, including if you:
- have liver problems
- have a history of a muscle disorder called rhabdomyolysis or myopathy
- have increased levels of creatine kinase in your blood
- have phenylketonuria (PKU). Isentress chewable tablets contain phenylalanine as part of the artificial sweetener, aspartame. The artificial sweetener may be harmful to people with PKU.
- receive kidney dialysis treatment
- are pregnant or plan to become pregnant. It is not known if Isentress or Isentress HD can harm your unborn baby.
- Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your doctor about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Isentress or Isentress HD.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if Isentress or Isentress HD can pass into your breast milk.
- Talk with your doctor about the best way to feed your baby.
Tell your doctor about all the medicines you take, including, prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines interact with Isentress and Isentress HD.
- Keep a list of your medicines to show your doctor and pharmacist.
- You can ask your doctor or pharmacist for a list of medicines that interact with Isentress and Isentress HD.
- Do not start taking a new medicine without telling your doctor. Your doctor can tell you if it is safe to take Isentress or Isentress HD with other medicines.
How should I take Isentress or Isentress HD?
- Take Isentress or Isentress HD exactly as prescribed by your doctor.
- Do not change your dose of Isentress or Isentress HD or stop your treatment without talking with your doctor first.
- Stay under the care of your doctor during treatment with Isentress or Isentress HD.
- Isentress film-coated tablets and Isentress HD film-coated tablets must be swallowed whole.
- Isentress chewable tablets may be chewed or swallowed whole.
- Do not switch between the film-coated tablet, the chewable tablet, or the oral suspension without talking with your doctor first.
- Do not switch between the Isentress 400 mg film-coated tablet and the Isentress HD 600 mg film-coated tablet if your prescribed dose is 1200 mg.
- Do not run out of Isentress or Isentress HD. The virus in your blood may increase and the virus may become harder to treat. Get a refill of your Isentress or Isentress HD from your doctor or pharmacy before you run out.
- Take Isentress or Isentress HD on a regular dosing schedule as instructed by your doctor. Do not miss doses.
- If you take too much Isentress or Isentress HD, call your doctor or go to the nearest hospital emergency room right away.
If Isentress for oral suspension is prescribed for your child, be sure to read the following information:
- Before giving the first dose of Isentress for oral suspension, read the Instructions for Use booklet that comes with Isentress for oral suspension for information about the correct way to mix and give a dose of Isentress for oral suspension to your child. Keep the booklet and follow it each time you prepare the medicine. Bring this booklet to your child’s appointments.
- Make sure your doctor shows you how to mix and give the right dose of Isentress for oral suspension to your child. If you have questions about how to mix or give Isentress for oral suspension, talk with your doctor or pharmacist.
- Give the dose of Isentress for oral suspension within 30 minutes of mixing.
- If your child does not take all of the prescribed dose or spits some of it out, call your doctor to find out what to do.
- Your child’s dose will change over time. Make sure you follow your doctor’s instructions. Your doctor will tell you if and when to stop giving Isentress to your child.
What are the possible side effects of Isentress or Isentress HD?
Isentress and Isentress HD can cause serious side effects including:
1. Severe skin reactions and allergic reactions. Some people who take Isentress or Isentress HD develop severe skin reactions and allergic reactions that can be serious, and may be life-threatening or lead to death.
- If you develop a rash, call your doctor right away.
- If you develop a rash with any of the following symptoms, stop using Isentress or Isentress HD and call your doctor or get medical help right away:
- fever
- generally ill feeling
- extreme tiredness
- muscle or joint aches
- blisters or sores in mouth
- blisters or peeling of the skin
- redness or swelling of the eyes
- swelling of the mouth, lips, or face
- problems breathing
Sometimes allergic reactions can affect body organs, such as your liver. Call your doctor right away if you have any of the following signs or symptoms of liver problems:
- yellowing of your skin or whites of your eyes
- dark or tea colored urine
- pale colored stools (bowel movements)
- nausea or vomiting
- loss of appetite
- pain, aching, or tenderness on the right side of your stomach area
2. Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your doctor right away if you start having new symptoms after starting your HIV-1 medicine.
The most common side effects of Isentress and Isentress HD include:
Less common side effects of Isentress and Isentress HD include:
- depression
- hepatitis
- genital herpes
- herpes zoster including shingles
- kidney failure
- kidney stones
- indigestion or stomach area pain
- vomiting
- suicidal thoughts and actions
- weakness
Tell your doctor right away if you get unexplained muscle pain, tenderness, or weakness during treatment with Isentress or Isentress HD. These may be signs of a rare serious muscle problem that can lead to kidney problems.
These are not all the possible side effects of Isentress and Isentress HD.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Isentress and Isentress HD
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet.
Do not use Isentress or Isentress HD for a condition for which it was not prescribed. Do not give Isentress or Isentress HD to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about Isentress or Isentress HD that is written for health professionals.
How should I store Isentress or Isentress HD?
Isentress and Isentress HD film-coated tablets:
- Store Isentress and Isentress HD film-coated tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Isentress and Isentress HD film-coated tablets in the original package with the bottle tightly closed.
- Keep the drying agent (desiccant) in the Isentress and Isentress HD bottle to protect from moisture.
Isentress chewable tablets:
- Store Isentress chewable tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Isentress chewable tablets in the original package with the bottle tightly closed.
- Keep the drying agent (desiccant) in the bottle to protect from moisture.
Isentress for oral suspension:
- Store Isentress for oral suspension at room temperature between 68°F to 77°F (20°C to 25°C).
- Store in the original container. Do not open the foil packet until ready for use.
Keep Isentress and all medicines out of the reach of children.
What are the ingredients in Isentress?
Active ingredient: raltegravir
Inactive ingredients:
Isentress 400 mg film-coated tablets: calcium phosphate dibasic anhydrous, hypromellose 2208, lactose monohydrate, magnesium stearate, microcrystalline cellulose, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate.
The film coating contains: black iron oxide, polyethylene glycol 3350, polyvinyl alcohol, red iron oxide, talc and titanium dioxide.
Isentress HD 600 mg film-coated tablets: croscarmellose sodium, hypromellose 2910, magnesium stearate, microcrystalline cellulose.
The film coating contains: ferrosoferric oxide, hypromellose 2910, iron oxide yellow, lactose monohydrate, triacetin and titanium dioxide.
The tablet may also contain trace amount of carnauba wax.
Isentress chewable tablets: ammonium hydroxide, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, magnesium stearate, mannitol, medium chain triglycerides, monoammonium glycyrrhizinate, natural and artificial flavors (orange, banana, and masking that contains aspartame), oleic acid, PEG 400, saccharin sodium, sodium citrate dihydrate, sodium stearyl fumarate, sorbitol, sucralose and yellow iron oxide. The 100 mg chewable tablet also contains red iron oxide.
Isentress for oral suspension: ammonium hydroxide, banana with other natural flavors, carboxymethylcellulose sodium, crospovidone, ethylcellulose 20 cP, fructose, hydroxypropyl cellulose, hypromellose 2910/6cP, macrogol/PEG 400, magnesium stearate, maltodextrin, mannitol, medium chain triglycerides, microcrystalline cellulose, monoammonium glycyrrhizinate, oleic acid, sorbitol, sucralose and sucrose.
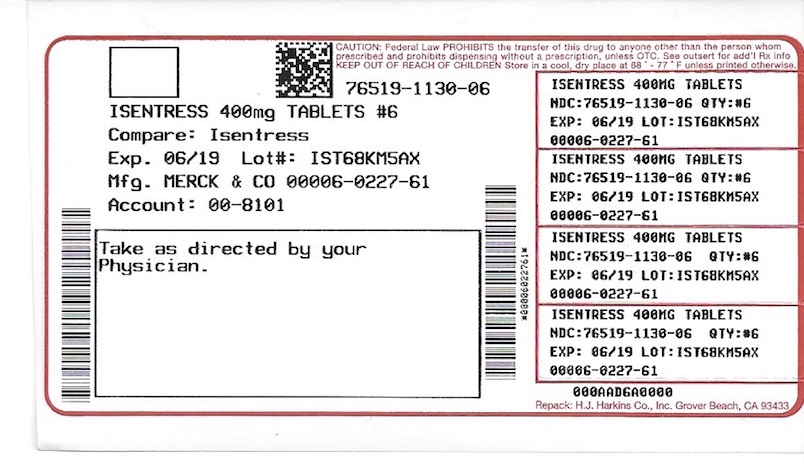
Label
PACKAGE LABEL. PRINICPAL DISPLAY PANEL