Invega Sustenna
Generic name: paliperidone (injection)
Drug class: Atypical antipsychotics
What is Invega Sustenna?
Invega Sustenna is a prescription medicine given by injection by a healthcare professional and used to treat:
- schizophrenia in adults, schizoaffective disorder in adults either alone or with other medicines such as mood stabilizers or antidepressants
It is not known if Invega Sustenna is safe and effective in children under 18 years of age.
Description
Invega Sustenna contains paliperidone palmitate. The active ingredient, paliperidone, is an atypical antipsychotic belonging to the chemical class of benzisoxazole derivatives. Invega Sustenna contains a racemic mixture of (+)- and (-)- paliperidone palmitate. The chemical name is (9RS)-3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimadin-9-yl hexadecanoate. Its molecular formula is C39H57FN4O4 and its molecular weight is 664.89. The structural formula is:
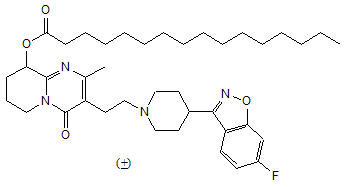
Paliperidone palmitate is very slightly soluble in ethanol and methanol, practically insoluble in polyethylene glycol 400 and propylene glycol, and slightly soluble in ethyl acetate.
What are the ingredients in Invega Sustenna?
Active ingredient: paliperidone palmitate
Inactive ingredients: polysorbate 20, polyethylene glycol 4000, citric acid monohydrate, sodium dihydrogen phosphate monohydrate, sodium hydroxide, and water for injection
What is the most important information I should know about Invega Sustenna?
Invega Sustenna can cause serious side effects, including:
- Increased risk of death in elderly people who are confused, have memory loss and have lost touch with reality (dementia-related psychosis). Invega Sustenna is not for treating dementia-related psychosis.
Who should not use Invega Sustenna?
Do not receive Invega Sustenna if you:
- are allergic to paliperidone, paliperidone palmitate, risperidone, or any of the ingredients in Invega Sustenna. See the end of this Patient Information guide for a complete list of ingredients in Invega Sustenna.
What should I tell my healthcare provider before using Invega Sustenna?
Before you receive Invega Sustenna, tell your healthcare provider about all your medical conditions, including if you:
- have had Neuroleptic Malignant Syndrome (NMS)
- have or have had heart problems, including a heart attack, heart failure, abnormal heart rhythm, or long QT syndrome
- have or have had low levels of potassium or magnesium in your blood
- have or have had uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia)
- have or have had kidney or liver problems
- have diabetes or have a family history of diabetes
- have had a low white blood cell count
- have had problems with dizziness or fainting or are being treated for high blood pressure
- have or have had seizures or epilepsy
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Invega Sustenna will harm your unborn baby.
- If you become pregnant while taking Invega Sustenna, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
- Infants born to women who are treated with Invega Sustenna may experience symptoms such as tremors, irritability, excessive sleepiness, eye twitching, muscle spasms, decreased appetite, difficulty breathing, or abnormal movement of arms and legs. Let your healthcare provider know if these symptoms occur.
- are breastfeeding or plan to breastfeed. Invega Sustenna can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive Invega Sustenna.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show to your healthcare provider or pharmacist when you get a new medicine.
How should I use Invega Sustenna?
- Follow your Invega Sustenna treatment schedule exactly as your healthcare provider tells you to.
- Your healthcare provider will tell you how much Invega Sustenna you will receive and when you will receive it.
- Invega Sustenna is given as an injection by your healthcare provider into the muscle (intramuscularly) of your arm or your buttocks.
- When you receive your first dose of Invega Sustenna you will need to get a second dose 1 week later. After that, you will only need to get a dose 1 time a month.
What should I avoid while using Invega Sustenna?
- Invega Sustenna may affect your ability to make decisions, think clearly, or react quickly. Do not drive, operate heavy machinery, or do other dangerous activities until you know how Invega Sustenna affects you.
- Avoid getting overheated or dehydrated.
What are the possible side effects of Invega Sustenna?
Invega Sustenna may cause serious side effects, including:
- stroke in elderly people (cerebrovascular problems) that can lead to death
- Neuroleptic Malignant Syndrome (NMS). NMS is a rare but very serious problem that can happen in people who receive Invega Sustenna. NMS can cause death and must be treated in a hospital. Call your healthcare provider right away if you become severely ill and have any of these symptoms:
- high fever
- severe muscle stiffness
- confusion
- loss of consciousness
- changes in your breathing, heartbeat and blood pressure
- problems with your heartbeat. These heart problems can cause death. Call your healthcare provider right away if you have any of these symptoms:
- passing out or feeling like you will pass out
- dizziness
- feeling as if your heart is pounding or missing beats
- uncontrolled movements of your tongue, face, mouth, or jaw (tardive dyskinesia)
- metabolic changes. Metabolic changes may include high blood sugar (hyperglycemia), diabetes mellitus and changes in the fat levels in your blood (dyslipidemia), and weight gain.
- low blood pressure and fainting
- changes in your blood cell counts
- high level of prolactin in your blood (hyperprolactinemia). Invega Sustenna may cause a rise in the blood levels of a hormone called prolactin (hyperprolactinemia) that may cause side effects including missed menstrual periods, leakage of milk from the breasts, development of breasts in men, or problems with erection.
- problems thinking clearly and moving your body
- seizures
- difficulty swallowing that can cause food or liquid to get into your lungs
- prolonged or painful erection lasting more than 4 hours. Call your healthcare provider or go to your nearest emergency room right away if you have an erection that lasts more than 4 hours.
- problems with control of your body temperature especially when you exercise a lot or spend time doing things that make you warm. It is important for you to drink water to avoid dehydration.
The most common side effects of Invega Sustenna include: injection site reactions, sleepiness or drowsiness, dizziness, feeling restlessness or needing to be constantly moving, abnormal muscle movements including tremor (shaking), shuffling, uncontrolled involuntary movements, and abnormal movements of your eyes.
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of Invega Sustenna. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How supplied/stored
INVEGA SUSTENNA is available as a white to off-white sterile aqueous extended-release suspension for intramuscular injection in dose strengths of 39 mg/0.25 mL, 78 mg/0.5 mL, 117 mg/0.75 mL, 156 mg/mL, and 234 mg/1.5 mL paliperidone palmitate in single-dose prefilled syringes. The single-use kit contains a prefilled syringe and 2 safety needles (a 1 ½-inch 22 gauge safety needle and a 1-inch 23 gauge safety needle).
| 39 mg paliperidone palmitate kit | (NDC 50458-560-01) |
| 78 mg paliperidone palmitate kit | (NDC 50458-561-01) |
| 117 mg paliperidone palmitate kit | (NDC 50458-562-01) |
| 156 mg paliperidone palmitate kit | (NDC 50458-563-01) |
| 234 mg paliperidone palmitate kit | (NDC 50458-564-01) |
Storage and Handling
Store at room temperature (25°C, 77°F); excursions between 15°C and 30°C (between 59°F and 86°F) are permitted. Do not mix with any other product or diluent.
Label
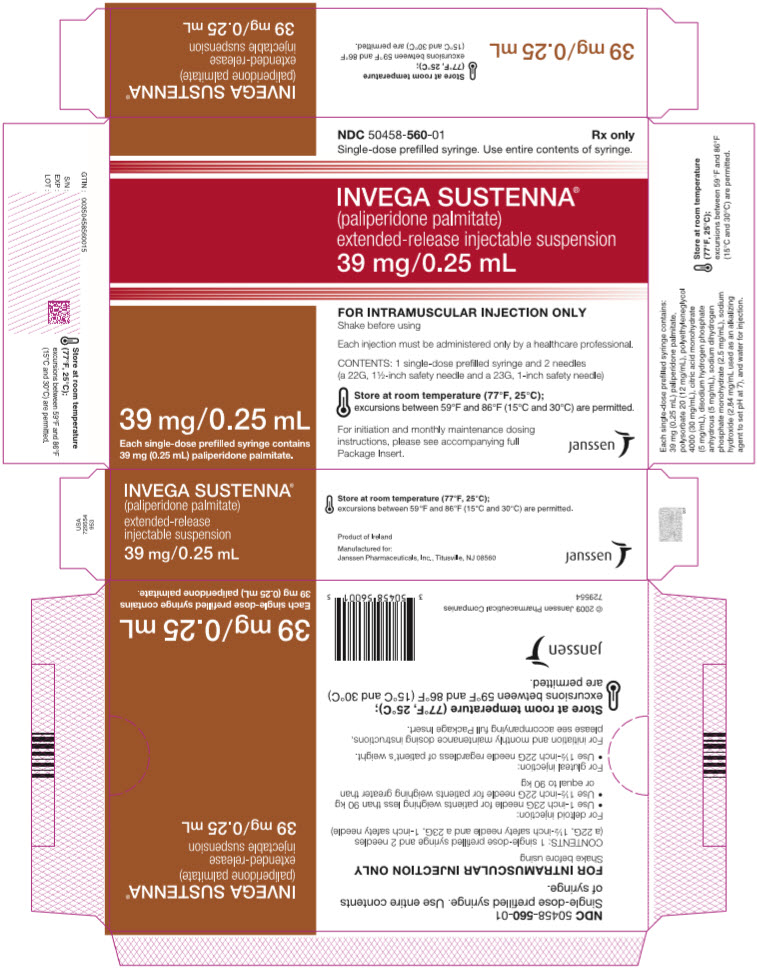
PRINCIPAL DISPLAY PANEL – 39 MG SYRINGE KIT
- NDC 50458-560-01
Rx only - Single-dose prefilled syringe. Use entire contents of syringe.
- INVEGA SUSTENNA®
- (paliperidone palmitate)
extended-release injectable suspension
39 mg/0.25 mL - 39 mg/0.25 mL
Each single-dose prefilled syringe contains
39 mg (0.25 mL) paliperidone palmitate. - FOR INTRAMUSCULAR INJECTION ONLY
Shake before using - Each injection must be administered only by a healthcare professional.
- CONTENTS: 1 single-dose prefilled syringe and 2 needles
(a 22G, 1½-inch safety needle and a 23G, 1-inch safety needle) - Store at room temperature (77°F, 25°C);
excursions between 59°F and 86°F (15°C and 30°C) are permitted. - For initiation and monthly maintenance dosing
instructions, please see accompanying full
Package Insert. - janssen
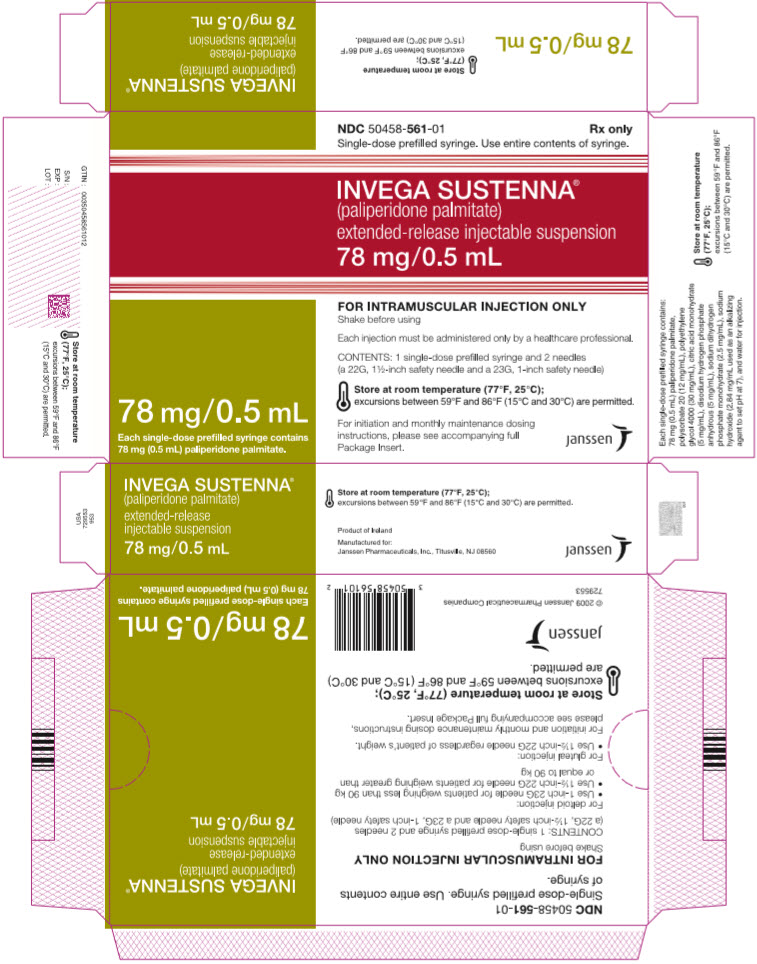
PRINCIPAL DISPLAY PANEL – 78 MG SYRINGE KIT
- NDC 50458-561-01
Rx only - Single-dose prefilled syringe. Use entire contents of syringe.
- INVEGA SUSTENNA®
- (paliperidone palmitate)
extended-release injectable suspension
78 mg/0.5 mL - 78 mg/0.5 mL
Each single-dose prefilled syringe contains
78 mg (0.5 mL) paliperidone palmitate. - FOR INTRAMUSCULAR INJECTION ONLY
Shake before using - Each injection must be administered only by a healthcare professional.
- CONTENTS: 1 single-dose prefilled syringe and 2 needles
(a 22G, 1½-inch safety needle and a 23G, 1-inch safety needle) - Store at room temperature (77°F, 25°C);
excursions between 59°F and 86°F (15°C and 30°C) are permitted. - For initiation and monthly maintenance dosing
instructions, please see accompanying full
Package Insert. - janssen
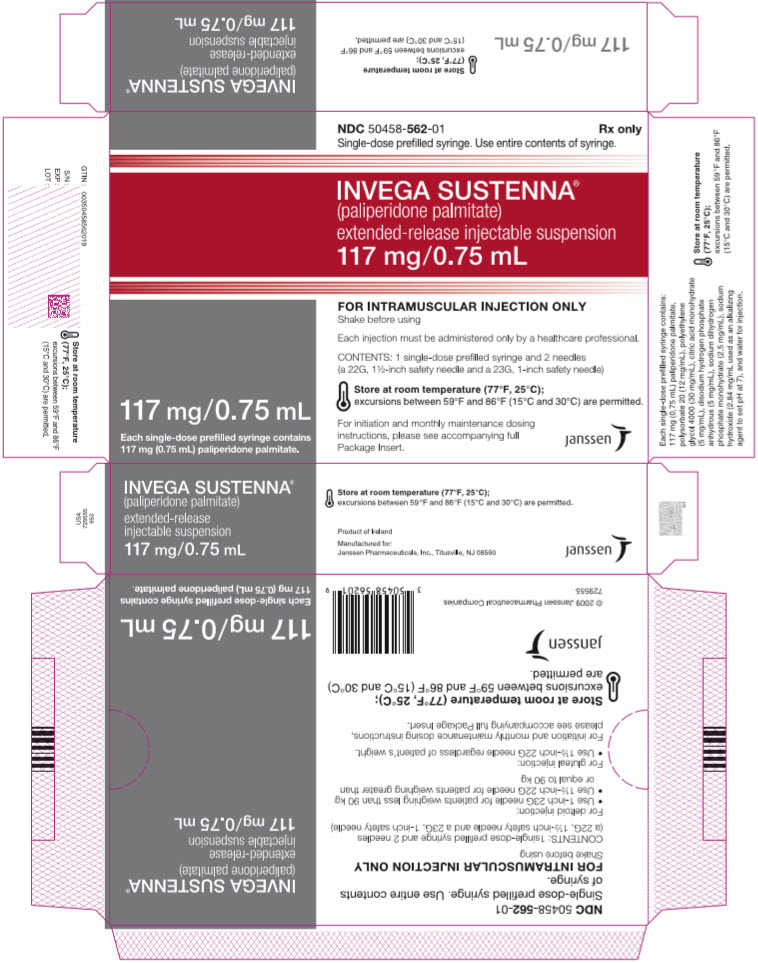
PRINCIPAL DISPLAY PANEL – 117 MG SYRINGE KIT
- NDC 50458-562-01
Rx only - Single-dose prefilled syringe. Use entire contents of syringe.
- INVEGA SUSTENNA®
- (paliperidone palmitate)
extended-release injectable suspension
117 mg/0.75 mL - 117 mg/0.75 mL
Each single-dose prefilled syringe contains
117 mg (0.75 mL) paliperidone palmitate. - FOR INTRAMUSCULAR INJECTION ONLY
Shake before using - Each injection must be administered only by a healthcare professional.
- CONTENTS: 1 single-dose prefilled syringe and 2 needles
(a 22G, 1½-inch safety needle and a 23G, 1-inch safety needle) - Store at room temperature (77°F, 25°C);
excursions between 59°F and 86°F (15°C and 30°C) are permitted. - For initiation and monthly maintenance dosing
instructions, please see accompanying full
Package Insert. - janssen
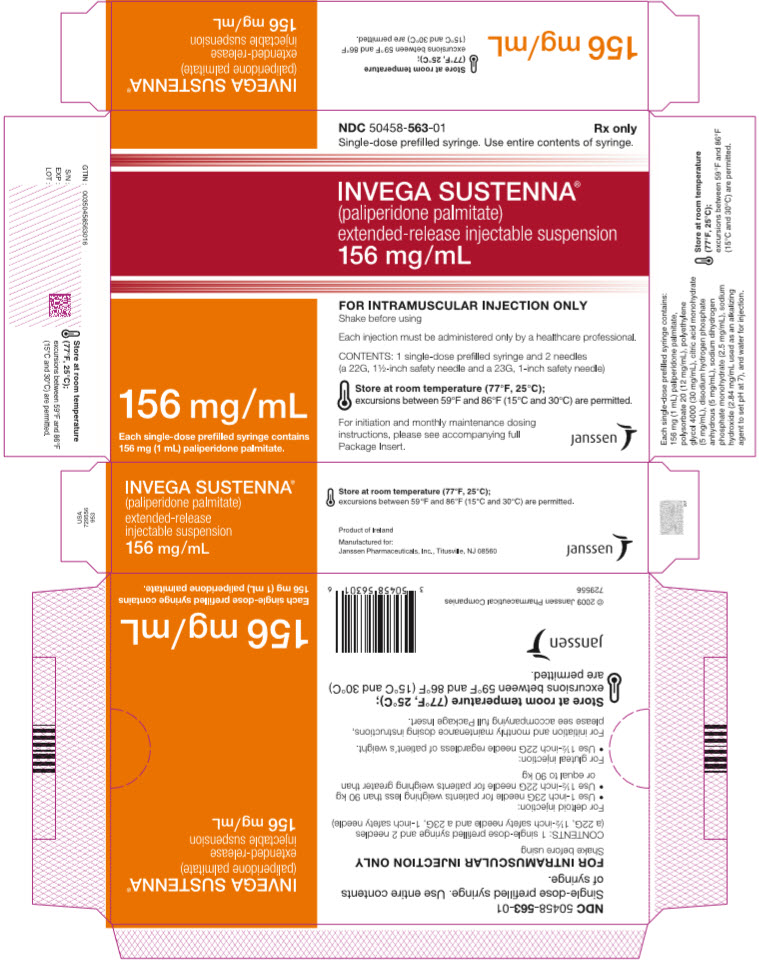
PRINCIPAL DISPLAY PANEL – 156 MG SYRINGE KIT
- NDC 50458-563-01
Rx only - Single-dose prefilled syringe. Use entire contents of syringe.
- INVEGA SUSTENNA®
- (paliperidone palmitate)
extended-release injectable suspension
156 mg/mL - 156 mg/mL
Each single-dose prefilled syringe contains
156 mg (1 mL) paliperidone palmitate. - FOR INTRAMUSCULAR INJECTION ONLY
Shake before using - Each injection must be administered only by a healthcare professional.
- CONTENTS: 1 single-dose prefilled syringe and 2 needles
(a 22G, 1½-inch safety needle and a 23G, 1-inch safety needle) - Store at room temperature (77°F, 25°C);
excursions between 59°F and 86°F (15°C and 30°C) are permitted. - For initiation and monthly maintenance dosing
instructions, please see accompanying full
Package Insert. - janssen
PRINCIPAL DISPLAY PANEL – 234 MG SYRINGE KIT
- NDC 50458-564-01
Rx only - Single-dose prefilled syringe. Use entire contents of syringe.
- INVEGA SUSTENNA®
- (paliperidone palmitate)
extended-release injectable suspension
234 mg/1.5 mL - 234 mg/1.5 mL
Each single-dose prefilled syringe contains
234 mg (1.5 mL) paliperidone palmitate. - FOR INTRAMUSCULAR INJECTION ONLY
Shake before using - Each injection must be administered only by a healthcare professional.
- CONTENTS: 1 single-dose prefilled syringe and 2 needles
(a 22G, 1½-inch safety needle and a 23G, 1-inch safety needle) - Store at room temperature (77°F, 25°C);
excursions between 59°F and 86°F (15°C and 30°C) are permitted. - For initiation and monthly maintenance dosing
instructions, please see accompanying full
Package Insert. - janssen

