Incruse Ellipta
Generic name: umeclidinium
Drug class: Anticholinergic bronchodilators
Medically reviewed by A Ras MD.
What is Incruse Ellipta?
Incruse Ellipta is an anticholinergic medicine (umeclidinium). Anticholinergic medicines such as umeclidinium help the muscles around the airways in your lungs stay relaxed to prevent symptoms such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
Incruse Ellipta is a prescription medicine used long term (chronic) to treat people with chronic obstructive pulmonary disease (COPD). COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
Incruse Ellipta is used as 1 inhalation 1 time each day to improve symptoms of COPD for better breathing and to reduce the number of flare-ups (the worsening of your COPD symptoms for several days).
Incruse Ellipta is not used to relieve sudden breathing problems and will not replace a rescue inhaler. Always have a rescue inhaler (an inhaled, short-acting bronchodilator) with you to treat sudden breathing problems. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you.
Incruse Ellipta should not be used in children. It is not known if Incruse Ellipta is safe and effective in children.
Description
INCRUSE ELLIPTA contains the active ingredient umeclidinium, an anticholinergic.
Umeclidinium bromide has the chemical name 1-[2-(benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-azoniabicyclo[2.2.2]octane bromide and the following chemical structure:
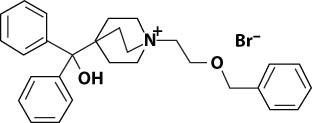
Umeclidinium bromide is a white powder with a molecular weight of 508.5, and the empirical formula is C29H34NO2•Br (as a quaternary ammonium bromide compound). It is slightly soluble in water.
INCRUSE ELLIPTA is a light grey and light green plastic inhaler containing a foil blister strip. Each blister on the strip contains a white powder mix of micronized umeclidinium bromide (74.2 mcg equivalent to 62.5 mcg of umeclidinium), magnesium stearate (75 mcg), and lactose monohydrate (to 12.5 mg). The lactose monohydrate contains milk proteins. After the inhaler is activated, the powder within the blister is exposed and ready for dispersion into the airstream created by the patient inhaling through the mouthpiece.
Under standardized in vitro test conditions, INCRUSE ELLIPTA delivers 55 mcg of umeclidinium per blister when tested at a flow rate of 60 L/min for 4 seconds.
In adult subjects with obstructive lung disease and severely compromised lung function (COPD with forced expiratory volume in 1 second/forced vital capacity [FEV1/FVC] <70% and FEV1 <30% predicted or FEV1 <50% predicted plus chronic respiratory failure), mean peak inspiratory flow through the ELLIPTA inhaler was 67.5 L/min (range: 41.6 to 83.3 L/min).
The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile.
Mechanism of Action
Umeclidinium is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptors at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine- and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of umeclidinium is predominantly a site-specific effect.
Who should not take Incruse Ellipta?
Do not use Incruse Ellipta if you:
- have a severe allergy to milk proteins. Ask your healthcare provider if you are not sure.
- are allergic to umeclidinium or any of the ingredients in Incruse Ellipta. See the end of this leaflet for a complete list of ingredients in Incruse Ellipta.
What should I tell my healthcare provider before taking Incruse Ellipta?
Before using Incruse Ellipta, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have eye problems such as glaucoma. Incruse Ellipta may make your glaucoma worse.
- have prostate or bladder problems, or problems passing urine. Incruse Ellipta may make these problems worse.
- are pregnant or plan to become pregnant. It is not known if Incruse Ellipta may harm your unborn baby.
- are breastfeeding. It is not known if the medicine in Incruse Ellipta passes into your breast milk and if it can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Incruse Ellipta and certain other medicines may interact with each other. This may cause serious side effects.
Especially tell your healthcare provider if you take:
- anticholinergics (including tiotropium, ipratropium, aclidinium)
- atropine
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Incruse Ellipta?
Read instructions for use that come with Incruse Ellipta.
- Do not use Incruse Ellipta unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Use Incruse Ellipta exactly as your healthcare provider tells you to use it. Do not use Incruse Ellipta more often than prescribed.
- Use 1 inhalation of Incruse Ellipta 1 time each day. Use Incruse Ellipta at the same time each day.
- If you miss a dose of Incruse Ellipta, take it as soon as you remember. Do not take more than 1 inhalation each day. Take your next dose at your usual time. Do not take 2 doses at 1 time.
- If you take too much Incruse Ellipta, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- Do not use other medicines that contain an anticholinergic for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are anticholinergic medicines.
- Do not stop using Incruse Ellipta unless told to do so by your healthcare provider.
- Talk to your healthcare provider right away if you stop using Incruse Ellipta.
- Incruse Ellipta does not relieve sudden symptoms of COPD and you should not take extra doses of Incruse Ellipta to relieve these sudden symptoms. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- Call your healthcare provider or get medical care right away if:
- your breathing problems get worse.
- you need to use your rescue inhaler more often than usual.
- your rescue inhaler does not work as well to relieve your symptoms.
What are the possible side effects of Incruse Ellipta?
Incruse Ellipta can cause serious side effects, including:
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop taking Incruse Ellipta and call your healthcare provider right away.
- serious allergic reactions (anaphylaxis). Stop using Incruse Ellipta and call your healthcare provider or go to the nearest emergency room right away if you get any of the following symptoms of a serious allergic reaction:
- rash
- swelling of your face, mouth, and tongue
- hives
- breathing problems
- severe itching
- new or worsening eye problems including acute narrow-angle glaucoma. You should have regular eye exams while using Incruse Ellipta. Acute narrow-angle glaucoma can cause permanent loss of vision if not treated.
Symptoms of acute narrow-angle glaucoma may include: - urinary retention. People who take Incruse Ellipta may develop new or worse urinary retention. Symptoms of urinary retention may include:
- difficulty urinating
- urinating frequently
- painful urination
- urination in a weak stream or drips
If you have these symptoms of urinary retention, stop taking Incruse Ellipta, and call your healthcare provider right away before taking another dose.
Common side effects of Incruse Ellipta include:
- upper respiratory tract infection
- stuffy or runny nose
- cough
- mouth and throat pain
- joint pain
- change in taste
- muscle pain
- tooth pain
- stomach pain
- bruising or dark areas of skin
- fast or irregular heartbeat
These are not all the possible side effects of Incruse Ellipta.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Incruse Ellipta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Incruse Ellipta for a condition for which it was not prescribed. Do not give Incruse Ellipta to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Incruse Ellipta that is written for health professionals.
How should I store Incruse Ellipta?
- Store Incruse Ellipta at room temperature between 68°F and 77°F (20°C and 25°C). Keep in a dry place away from heat and sunlight.
- Store Incruse Ellipta in the unopened tray and only open when ready for use.
- Safely throw away Incruse Ellipta in the trash 6 weeks after you open the tray or when the counter reads “0”, whichever comes first. Write the date you open the tray on the label on the inhaler.
- Keep Incruse Ellipta and all medicines out of the reach of children.
What are the ingredients in Incruse Ellipta?
Active ingredient: umeclidinium
Inactive ingredients: lactose monohydrate (contains milk proteins), magnesium stearate
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0873-10
- INCRUSE ELLIPTA
- (umeclidinium inhalation powder)
- 62.5 mcg
- Rx Only
- FOR ORAL INHALATION ONLY
- Each blister contains 62.5 mcg of umeclidinium, magnesium stearate, and lactose monohydrate.
- 1 ELLIPTA Inhaler containing 1 Foil Strip of 30 Blisters
- Made in Singapore
- ©2022 GSK group of companies or its licensor
- 62000000074512 Rev. 1/22
-
SRC: NLM .
