Igalmi dosage and instructions
- Generic: dexmedetomidine
- Dosage Form sublingual film, for sublingual or buccal use
Medically reviewed by A Ras MD. Last updated April 8, 2022
Igalmi Description
IGALMI contains dexmedetomidine, an alpha2-adrenergic receptor agonist, present as dexmedetomidine hydrochloride, the S-enantiomer of medetomidine chemically described as 4-[(1S)-1(2, 3-dimethylphenyl) ethyl]-1H-imidazole hydrochloride. The empirical formula is C13H16N2•HCl with a molecular weight of 236.7 g/mol. The structural formula of dexmedetomidine hydrochloride is: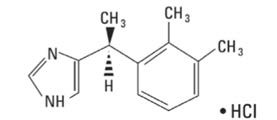
Dexmedetomidine hydrochloride is a white or almost white powder that is freely soluble in water and has a pKa of 7.1. Its partition coefficient in octanol/water at pH 7.4 is 2.89.
IGALMI is for sublingual or buccal use. Each IGALMI sublingual film contains 120 mcg or 180 mcg of dexmedetomidine equivalent to 141.8 mcg and 212.7 mcg of dexmedetomidine hydrochloride, respectively.
IGALMI contains the following inactive ingredients: FD&C Blue #1 colorant, hydroxypropyl cellulose, peppermint oil, polyethylene oxide, and sucralose.
Indications and usage
IGALMI is indicated for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults.
Limitations of Use
The safety and effectiveness of IGALMI have not been established beyond 24 hours from the first dose
Dosage forms and strength
IGALMI is a blue rectangular sublingual film containing on its surface two darker blue spots in dose strengths of 120 mcg and 180 mcg.
Dosage and Instructions
Important Recommendations Prior to Initiating IGALMI and During Therapy
IGALMI should be administered under the supervision of a healthcare provider. A healthcare provider should monitor vital signs and alertness after IGALMI administration to prevent falls and syncope.
IGALMI is for sublingual or buccal administration. Do not chew or swallow IGALMI. Do not eat or drink for at least 15 minutes after sublingual administration, or at least one hour after buccal administration.¶
Recommended Dosage
Table 1 includes dosage recommendations for IGALMI based on agitation severity for adults, patients with hepatic impairment, and geriatric patients. Lower dosages are recommended for patients with hepatic impairment and geriatric patients.
If agitation persists after the initial dose, up to two additional doses may be administered at least two hours apart. The dosage recommendations for additional doses vary depending upon the patient population and agitation severity (see Table 1). Assess vital signs including orthostatic measurements prior to the administration of any subsequent doses.
Due to risk of hypotension, additional half-doses are not recommended in patients with systolic blood pressure (SBP) less than 90 mmHg, diastolic blood pressure (DBP) less than 60 mmHg, heart rate (HR) less than 60 beats per minute, or postural decrease in SBP ≥ 20 mmHg or in DBP ≥ 10 mmHg.
Table 1: Dosage Recommendations for IGALMI in Adults, Adult Patients with Hepatic Impairment, and Geriatric Patients with Agitation Associated with Schizophrenia or Bipolar I or II Disorder
| Patient Population | Agitation Severity | Initial
Dose* |
Optional 2nd/3rd Doses* | Maximum
Recommended Total Daily Dosage |
| Adults | Mild or Moderate | 120 mcg | 60 mcg | 240 mcg |
| Severe | 180 mcg | 90 mcg | 360 mcg | |
| Patients with Mild or
Moderate Hepatic Impairment** |
Mild or Moderate | 90 mcg | 60 mcg | 210 mcg |
| Severe | 120 mcg | 60 mcg | 240 mcg | |
| Patients with Severe
Hepatic Impairment** |
Mild or Moderate | 60 mcg | 60 mcg | 180 mcg |
| Severe | 90 mcg | 60 mcg | 210 mcg | |
| Geriatric Patients
(≥ 65 years old) |
Mild, Moderate, or Severe | 120 mcg | 60 mcg | 240 mcg |
* IGALMI 120 mcg and 180 mcg dosage strengths may be cut in half to obtain the 60 mcg and 90 mcg doses, respectively
** Hepatic impairment: Mild (Child-Pugh Class A); Moderate (Child-Pugh Class B); Severe (Child-Pugh Class C)
Preparation and Administration Instructions
Keep IGALMI in the foil pouch until ready to administer. IGALMI should be immediately administered once the pouch is opened and the dose prepared.
Prepare and administer IGALMI under the supervision of a healthcare provider as follows:
| Healthcare Professional: Prepare IGALMI Dose for Patient | ||
| 1 | Open the sealed foil pouch by tearing straight across at the notch.
|
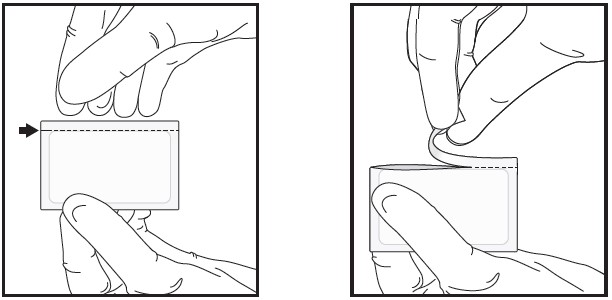 |
| • Perform Steps 2a, 2b, 2c and 2d only if a 60 mcg or 90 mcg dose (half of a film) is needed, then proceed to Step 3.
• If administering a full dose (1 film), proceed directly to Step 3.
|
||
| 2a | Remove the film from the pouch with clean dry hands.
|
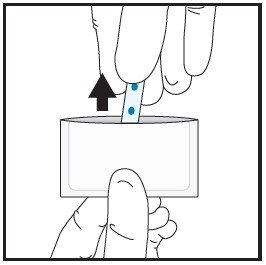 |
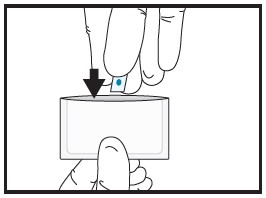
| 2b | Cut the film in half between the dots with clean, dry scissors.
|
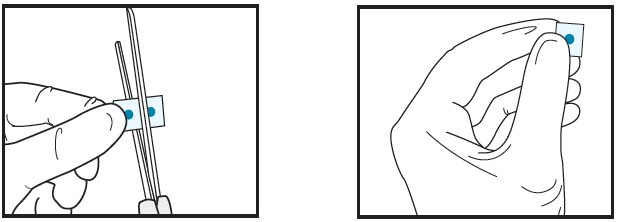 |
| 2c | Discard unused half in waste container.
|
|
| 2d | Place the half film for
administration to the patient back into the pouch. |
 |
| 3 | Immediately give the pouch to the patient. | 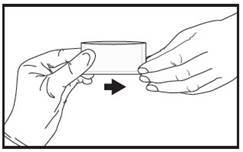 |
| 4 | Instruct patient to remove the film from the pouch with clean dry hands. | 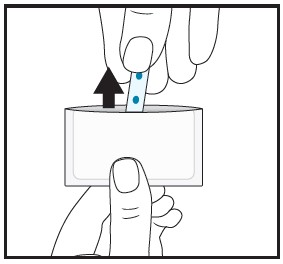 |
| 5 | For sublingual administration: Instruct patient to place film under the tongue. The film will stick in place.
Note: Patient may not eat or drink for 15 minutes after sublingual administration.
|
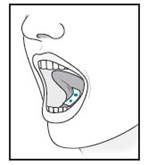 |
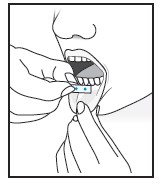
| For buccal administration: Instruct patient to place film behind lower lip. The film will stick in place.
Note: Patient may not eat or drink for one hour after buccal administration. |
 |
|
| 6 | Instruct patient to:
• Close their mouth. • Allow the film to dissolve. • Do not chew or swallow the film. |
|
