Ibrance
Generic name: palbociclib
Drug class: CDK 4/6 inhibitors
Medically reviewed by A Ras MD.
What is Ibrance?
Ibrance is a prescription medicine used in adults to treat hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body (metastatic) in combination with an aromatase inhibitor as the first hormonal based therapy in postmenopausal women or in men, or fulvestrant with disease progression following hormonal therapy.
It is not known if Ibrance is safe and effective in children.
Description
IBRANCE capsules for oral administration contain 125 mg, 100 mg, or 75 mg of palbociclib, a kinase inhibitor. The molecular formula for palbociclib is C24H29N7O2. The molecular weight is 447.54 daltons. The chemical name is 6-acetyl-8-cyclopentyl-5-methyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}pyrido[2,3-d]pyrimidin-7(8H)-one, and its structural formula is:
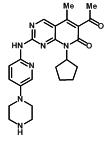
Palbociclib is a yellow to orange powder with pKa of 7.4 (the secondary piperazine nitrogen) and 3.9 (the pyridine nitrogen). At or below pH 4, palbociclib behaves as a high-solubility compound. Above pH 4, the solubility of the drug substance reduces significantly.
Inactive ingredients: Microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, colloidal silicon dioxide, magnesium stearate, and hard gelatin capsule shells. The light orange, light orange/caramel, and caramel opaque capsule shells contain gelatin, red iron oxide, yellow iron oxide, and titanium dioxide; the printing ink contains shellac, titanium dioxide, ammonium hydroxide, propylene glycol, and simethicone.
Mechanism of Action
Palbociclib is an inhibitor of cyclin-dependent kinases (CDK) 4 and 6. Cyclin D1 and CDK4/6 are downstream of signaling pathways which lead to cellular proliferation. In vitro, palbociclib reduced cellular proliferation of estrogen receptor (ER)-positive breast cancer cell lines by blocking progression of the cell from G1 into S phase of the cell cycle.
Treatment of breast cancer cell lines with the combination of palbociclib and antiestrogens leads to decreased retinoblastoma (Rb) protein phosphorylation resulting in reduced E2F expression and signaling, and increased growth arrest compared to treatment with each drug alone. In vitro treatment of ER-positive breast cancer cell lines with the combination of palbociclib and antiestrogens led to increased cell senescence compared to each drug alone, which was sustained for up to 6 days following palbociclib removal and was greater if antiestrogen treatment was continued. In vivo studies using a patient-derived ER-positive breast cancer xenograft model demonstrated that the combination of palbociclib and letrozole increased the inhibition of Rb phosphorylation, downstream signaling, and tumor growth compared to each drug alone.
Human bone marrow mononuclear cells treated with palbociclib in the presence or absence of an anti-estrogen in vitro did not become senescent and resumed proliferation following palbociclib withdrawal.
What is the most important information I should know about Ibrance?
Ibrance may cause serious side effects, including:
Low white blood cell counts (neutropenia). Low white blood cell counts are very common when taking Ibrance and may cause serious infections that can lead to death. Your healthcare provider should check your white blood cell counts before and during treatment.
If you develop low white blood cell counts during treatment with Ibrance, your healthcare provider may stop your treatment, decrease your dose, or may tell you to wait to begin your treatment cycle. Tell your healthcare provider right away if you have signs and symptoms of low white blood cell counts or infections such as fever and chills.
Lung problems (pneumonitis). Ibrance may cause severe or life-threatening inflammation of the lungs during treatment that can lead to death. Tell your healthcare provider right away if you have any new or worsening symptoms, including:
- trouble breathing or shortness of breath
- cough with or without mucus
- chest pain
See “What are the possible side effects of Ibrance?” for more information about side effects.
What should I tell my healthcare provider before taking Ibrance?
Before you take Ibrance, tell your healthcare provider if you:
- have fever, chills, or any other signs or symptoms of infection.
- have liver or kidney problems.
- have any other medical conditions.
- are pregnant, or plan to become pregnant. Ibrance can harm your unborn baby.
- Females who are able to become pregnant should use effective birth control during treatment and for at least 3 weeks after the last dose of Ibrance.
- Males with female partners who can become pregnant should use effective birth control during treatment with Ibrance for at least 3 months after the last dose of Ibrance.
- Talk to your healthcare provider about birth control methods that may be right for you during this time.
- If you become pregnant or think you are pregnant, tell your healthcare provider right away.
- are breastfeeding or plan to breastfeed. It is not known if Ibrance passes into your breast milk. Do not breastfeed during treatment with Ibrance and for 3 weeks after the last dose.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Ibrance and other medicines may affect each other causing side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take Ibrance?
- Take Ibrance exactly as your healthcare provider tells you.
- Take Ibrance with food.
- Ibrance should be taken at about the same time each day.
- Swallow Ibrance capsules whole. Do not chew, crush or open Ibrance capsules before swallowing them.
- Do not take any Ibrance capsules that are broken, cracked, or that look damaged.
- Avoid grapefruit and grapefruit products during treatment with Ibrance. Grapefruit may increase the amount of Ibrance in your blood.
- Do not change your dose or stop taking Ibrance unless your healthcare provider tells you.
- If you miss a dose of Ibrance or vomit after taking a dose of Ibrance, do not take another dose on that day. Take your next dose at your regular time.
- If you take too much Ibrance, call your healthcare provider right away or go to the nearest hospital emergency room.
What are the possible side effects of Ibrance?
Ibrance may cause serious side effects. See “What is the most important information I should know about Ibrance?”
Common side effects of Ibrance when used with either letrozole or fulvestrant include:
- Low red blood cell counts and low platelet counts are common with Ibrance. Call your healthcare provider right away if you develop any of these symptoms during treatment:
Ibrance may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider about family planning options before starting Ibrance if this is a concern for you.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Ibrance.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Ibrance
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Ibrance for a condition for which it was not prescribed. Do not give Ibrance to other people, even if they have the same symptoms you have. It may harm them.
If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for more information about Ibrance that is written for health professionals.
How should I store Ibrance?
- Store Ibrance at 68 °F to 77 °F (20 °C to 25 °C).
Keep Ibrance and all medicines out of the reach of children.
What are the ingredients in Ibrance?
Active ingredient: palbociclib
Inactive ingredients: microcrystalline cellulose, lactose monohydrate, sodium starch glycolate, colloidal silicon dioxide, magnesium stearate, and hard gelatin capsule shells.
The light orange, light orange/caramel and caramel opaque capsule shells contain: gelatin, red iron oxide, yellow iron oxide, and titanium dioxide.
The printing ink contains: shellac, titanium dioxide, ammonium hydroxide, propylene glycol, and simethicone.
Label
PRINCIPAL DISPLAY PANEL – 75 MG CAPSULE BOTTLE LABEL
- NDC 0069-0187-21
- Pfizer
- Ibrance®
(palbociclib)
capsules - 75 mg
- 21 Capsules
- Rx only

PRINCIPAL DISPLAY PANEL – 100 MG CAPSULE BOTTLE LABEL
- NDC 0069-0188-21
- Pfizer
- Ibrance®
(palbociclib)
capsules - 100 mg
- 21 Capsules
- Rx only


PRINCIPAL DISPLAY PANEL – 125 MG CAPSULE BOTTLE LABEL


