Hepsera
Generic name: adefovir
Drug class: Nucleoside reverse transcriptase inhibitors (NRTIs)
What is Hepsera?
Hepsera is a medicine used to treat people 12 years of age and older with chronic (long-lasting) infections with active hepatitis B virus.
Hepsera is not for use in children under 12 years of age.
Hepsera may lower the amount of hepatitis B virus (HBV) in your body. Hepsera may improve the condition of your liver.
Description
HEPSERA® is the tradename for adefovir dipivoxil, a diester prodrug of adefovir. Adefovir is an acyclic nucleotide analog with activity against human hepatitis B virus (HBV).
The chemical name of adefovir dipivoxil is 9-[2-[[bis[(pivaloyloxy)methoxy]-phosphinyl]-methoxy]ethyl]adenine. It has a molecular formula of C20H32N5O8P, a molecular weight of 501.48 and the following structural formula:
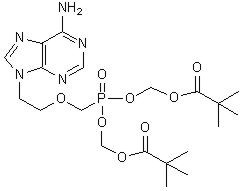
Adefovir dipivoxil is a white to off-white crystalline powder with an aqueous solubility of 19 mg/mL at pH 2.0 and 0.4 mg/mL at pH 7.2. It has an octanol/aqueous phosphate buffer (pH 7) partition coefficient (log p) of 1.91.
HEPSERA tablets are for oral administration. Each tablet contains 10 mg of adefovir dipivoxil and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, pregelatinized starch, and talc.
What is the most important information I should know about Hepsera?
Hepsera can cause serious side effects, including:
- Worsening of hepatitis B infection. Your hepatitis B (HBV) infection may become worse (flare-up) if you take Hepsera and then stop taking it. A “flare-up” is when your HBV infection suddenly returns in a worse way than before.
- Do not run out of Hepsera. Refill your prescription or talk to your doctor before your Hepsera is all gone.
- Do not stop taking Hepsera without first talking with your doctor.
- If you stop taking Hepsera, your doctor will need to check your health often and do blood tests regularly to check your HBV infection for at least several months.
- Hepsera may cause severe kidney problems. Severe kidney problems can happen in anyone who takes Hepsera, but certain people may have a higher risk of severe kidney problems with Hepsera, including:
- people who already have kidney problems or are at an increased risk for kidney problems, and
- people who take certain medicines that can cause kidney problems. Ask your doctor whether any of the medicines you currently take can cause kidney problems.
Your doctor should do blood tests to check your kidney function during treatment with Hepsera.
If you have kidney problems before you start taking Hepsera your doctor may change your dose of Hepsera. If you develop kidney problems during treatment, your doctor may need to change your dose of Hepsera or may stop your treatment. - HIV resistance. Your doctor may test you for HIV-1 infection before your start Hepsera. If you have both HBV and HIV-1 and you only take Hepsera, the HIV-1 virus may develop resistance and become harder to treat.
- Build-up of acid in your blood (lactic acidosis). Lactic acidosis can happen in some people who take Hepsera. Lactic acidosis is a serious medical emergency that can lead to death.
Call your doctor right away if you get any of the following symptoms which could be signs of lactic acidosis - Severe liver problems. In rare cases, severe liver problems can happen that lead to death. Call your doctor right away if you get any of the following signs or symptoms of liver problems.
- your skin or the white part of your eyes turns yellow (jaundice).
- dark or “tea-colored” urine
- light-colored stools (bowel movements)
- nausea
- loss of appetite for several days or longer
- pain, aching, or tenderness on the right side of your stomach-area
You may be more likely to get lactic acidosis or serious liver problems if you are female, are very overweight (obese), or have been taking nucleoside analog medicines for a long time.
Who should not take Hepsera?
Do not take Hepsera if you are allergic to any of the ingredients in Hepsera. See the end of this leaflet for a complete list of the ingredients in Hepsera.
What should I tell my healthcare provider before taking Hepsera?
Before taking Hepsera, tell your doctor about all of your medical conditions, including if you:
- have or had kidney problems. Your dose and schedule of Hepsera may need to be changed.
- are pregnant or plan to become pregnant. It is not known if Hepsera will harm your unborn baby.
Pregnancy Registry: There is a pregnancy registry for women who take Hepsera during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk with your doctor about how you can take part in this registry. - are breastfeeding. It is not known if Hepsera passes into your breast milk. Talk with your doctor about the best way to feed your baby during treatment with Hepsera.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may affect how Hepsera works, especially medicines that affect how your kidneys work. Hepsera can affect how your other medicines work. Your dose of Hepsera and the other medicines may need to be changed. Do not start a new medicine without telling your doctor. Your doctor can tell you if it is safe to take Hepsera with other medicines.
Especially tell your doctor if you take a medicine that contains tenofovir disoproxil fumarate or tenofovir alafenamide.
How should I take Hepsera?
- Take Hepsera exactly as your doctor tells you to take it.
- Your doctor will tell you how much Hepsera to take, and when and how often to take it.
- Do not change your dose or stop Hepsera without talking to your doctor. Your hepatitis may get worse if you change doses or stop.
- Take Hepsera at the same time each day that your doctor tells you, to avoid missing doses.
- Take Hepsera with or without food.
- Stay under the care of your doctor during treatment with Hepsera.
- When your Hepsera supply gets low, call your doctor or pharmacy for a refill. Do not run out of Hepsera.
- If you take too much Hepsera, call your doctor or go to the nearest hospital emergency room right away.
- It is not known how long you should take Hepsera. You and your doctor will need to decide when it is best for you to stop taking Hepsera. Some people get worsening of their hepatitis B infection when they stop taking Hepsera.
See “What is the most important information I should know about Hepsera?”.
What are the possible side effects of Hepsera?
Hepsera can cause serious side effects. See “What is the most important information I should know about Hepsera?”
The most common side effects of Hepsera are weakness, headache, stomach pain, and nausea.
These are not all the possible side effects of Hepsera. For more information, ask your doctor or pharmacist.
Call your doctor for advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
General information about the safe and effective use of Hepsera
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Hepsera for a condition for which it was not prescribed. Do not give Hepsera to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Hepsera that is written for health professionals.
How should I store Hepsera?
- Store Hepsera at room temperature, between 68°F to 77°F (20°C to 25°C).
- Keep Hepsera in its original container.
- Do not use Hepsera if the seal over the bottle is broken or missing when you receive it.
Keep Hepsera and all medicines out of the reach of children.
What are the ingredients in Hepsera?
Active Ingredient: adefovir dipivoxil
Inactive Ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, pregelatinized starch, and talc
Label
PRINCIPAL DISPLAY PANEL – 30 CT Representative Label
- HEPSERA® (ADEFOVIR DIPIVOXIL 10 MG)
TABLETS, 30 CT – BRITESTOCK - STORE BELOW 25°C
- Part Number: 7000003
- Lot Number: 1005247
- Quantity: 080
- CONTENTS MADE IN GERMANY
MANUFACTURED FOR:
GILEAD SCIENCES LIMITED
CORK, IRELAND - 4000738.00

