Gralise
Generic name: gabapentin
Drug class: Gamma-aminobutyric acid analogs
Medically reviewed by A Ras MD.
What is Gralise?
Gralise is a prescription medicine used in adults, 18 years and older, to treat pain from damaged nerves (neuropathic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection).
It is not known if Gralise is safe and effective in people with seizure problems (epilepsy). It is not known if Gralise is safe and effective in children under 18 years of age with postherpetic pain. Gralise is not interchangeable with other gabapentin products.
Description
Gabapentin is 1-(aminomethyl)cyclohexaneacetic acid; γ-amino-2-cyclohexyl-butyric acid with a molecular formula of C9H17NO2 and a molecular weight of 171.24.
The structural formula is:
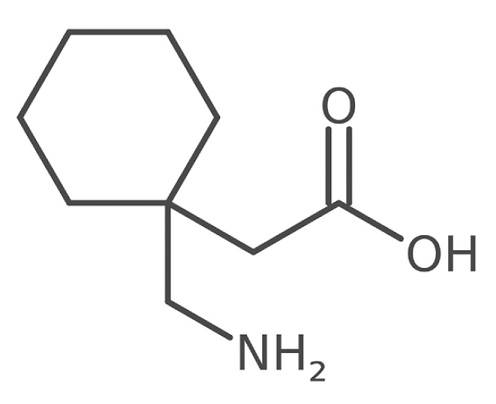
Gabapentin is a white to off-white crystalline solid with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water and acidic and basic solutions. The log of the partition coefficient (n-octanol/ 0.05M phosphate buffer) at pH 7.4 is -1.25.
GRALISE is supplied as tablets containing 300 mg or 600 mg of gabapentin. GRALISE tablets swell in gastric fluid and gradually release gabapentin. Each 300 mg tablet contains the inactive ingredients copovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene oxide, and Opadry® II white. Opadry® II white contains polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol 3350, and lecithin (soya). Each 600 mg tablet contains the inactive ingredients copovidone, hypromellose, magnesium stearate, polyethylene oxide, and Opadry® II beige. Opadry® II beige contains polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol 3350, iron oxide yellow, and iron oxide red.
Mechanism of Action
The mechanism of action by which gabapentin exerts its analgesic action is unknown but in animal models of analgesia, gabapentin prevents allodynia (pain-related behavior in response to a normally innocuous stimulus) and hyperalgesia (exaggerated response to painful stimuli). Gabapentin prevents pain-related responses in several models of neuropathic pain in rats and mice (e.g., spinal nerve ligation models, spinal cord injury model, acute herpes zoster infection model). Gabapentin also decreases pain-related responses after peripheral inflammation (carrageenan footpad test, late phase of formulin test), but does not alter immediate pain-related behaviors (rat tail flick test, formalin footpad acute phase). The relevance of these models to human pain is not known.
Gabapentin is structurally related to the neurotransmitter GABA (gamma-aminobutyric acid), but it does not modify GABAA or GABAB radioligand binding, it is not converted metabolically into GABA or a GABA agonist, and it is not an inhibitor of GABA uptake or degradation.
In radioligand binding assays at concentrations up to 100 μM, gabapentin did not exhibit affinity for a number of other receptor sites, including benzodiazepine, glutamate, N-methyl-D-aspartate (NMDA), quisqualate, kainate, strychnine-insensitive or strychnine-sensitive glycine; alpha 1, alpha 2, or beta adrenergic; adenosine A1 or A2; cholinergic, muscarinic, or nicotinic; dopamine D1 or D2; histamine H1; serotonin S1 or S2; opiate mu, delta, or kappa; cannabinoid 1; voltage-sensitive calcium channel sites labeled with nitrendipine or diltiazem; or at voltage-sensitive sodium channel sites labeled with batrachotoxinin A20-alpha-benzoate. Gabapentin did not alter the cellular uptake of dopamine, noradrenaline, or serotonin.
In vitro studies with radiolabeled gabapentin have revealed a gabapentin binding site in areas of rat brain including neocortex and hippocampus. A high-affinity binding protein in animal brain tissue has been identified as an auxiliary subunit of voltage-activated calcium channels. However, functional correlates of gabapentin binding, if any, remain to be elucidated. It is hypothesized that gabapentin antagonizes thrombospondin binding to α2δ-1 as a receptor involved in excitatory synapse formation and suggested that gabapentin may function therapeutically by blocking new synapse formation.
What is the most important information I should know about Gralise?
Do not stop taking Gralise without first talking with your healthcare provider. Stopping Gralise suddenly can cause serious problems.
Like other antiepileptic drugs, gabapentin, the active ingredient in Gralise, may cause suicidal thoughts or actions in a very small number of people, about 1 in 500. However, it is not known if Gralise is safe and effective in people with seizure problems (epilepsy). Therefore, Gralise should not be used in place of other gabapentin products.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop taking Gralise without first talking with your healthcare provider.
- Stopping Gralise suddenly can cause serious problems.
Who should not take Gralise?
Do not take Gralise if you are allergic to gabapentin or any of the ingredients in Gralise. See the end of this Medication Guide for a complete list of ingredients in Gralise.
What should I tell my healthcare provider before taking Gralise?
Before taking Gralise, tell your healthcare provider if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have seizures
- have kidney problems or get kidney dialysis
- are pregnant or plan to become pregnant. It is not known if Gralise can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking Gralise. You and your healthcare provider will decide if you should take Gralise while you are pregnant.
- If you become pregnant while taking Gralise, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic drugs, including gabapentin, the active ingredient in Gralise, during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. Gralise can pass into your breast milk. You and your healthcare provider should decide how you will feed your baby while you take Gralise.
Tell your healthcare provider about all the medicines you take including prescription and non-prescription medicines, vitamins or herbal supplements.
Taking Gralise with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Gralise?
- Take Gralise exactly as prescribed. Your healthcare provider will tell you how much Gralise to take and when to take it. Take Gralise at the same time each day.
- Do not change your dose or stop taking Gralise without talking with your healthcare provider. If you stop taking Gralise suddenly, you may experience side effects. Talk with your healthcare provider about how to stop Gralise slowly.
- Take Gralise with food one time each day with your evening meal.
- Take Gralise tablets whole. Do not split, crush, or chew Gralise tablets before swallowing.
- Your healthcare provider may change your dose of Gralise. Do not change your dose of Gralise without talking to your healthcare provider.
- If you miss a dose, take it as soon as you remember with food. If it is almost time for your next dose, just skip the missed dose. Take the next dose at your regular time. Do not take two doses at the same time.
- If you take too much Gralise, call your healthcare provider or poison control center, or go to the nearest emergency room right away.
- If you are taking an antacid containing aluminum hydroxide and magnesium hydroxide, it is recommended that Gralise be taken at least 2 hours following administration of the antacid.
What should I avoid while taking Gralise?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking Gralise without first talking to your healthcare provider. Taking Gralise with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not operate heavy machines or do other dangerous activities until you know how Gralise affects you. Gralise can slow your thinking and motor skills.
What are the possible side effects of Gralise?
The most common side effect of Gralise is, nausea, dizziness.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of Gralise. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Gralise
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Gralise for a condition for which it was not prescribed. Do not give Gralise to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about Gralise. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Gralise that is written for health professionals.
For more information about Gralise, call 1-866-458-6389.
How should I store Gralise?
Store Gralise at 59°F to 86°F (15°C to 30°C)
- Keep Gralise and all medicines out of the reach of children.
What are the ingredients in Gralise?
Active ingredient: gabapentin
Inactive ingredients:
300 mg tablet: copovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene oxide, and Opadry II white. Opadry II white contains polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol 3350, and lecithin (soya).
600 mg tablet: copovidone, hypromellose, magnesium stearate, polyethylene oxide, and Opadry II beige. Opadry II beige contains polyvinyl alcohol, titanium dioxide, talc, polyethylene glycol 3350, iron oxide yellow, and iron oxide red.
Label
PRINCIPAL DISPLAY PANEL
- NDC 52427-803-90
- ONCE-DAILY
Gralise®
(gabapentin) tablets - 300 mg
- Do not use Gralise interchangeably with other gabapentin products.
- PHARMACIST: Dispense the accompanying Medication Guide to each patient.

PRINCIPAL DISPLAY PANEL
- NDC 52427-806-90
- ONCE-DAILY
Gralise®
(gabapentin) tablets - 600 mg
- Do not use Gralise interchangeably with other gabapentin products.
- PHARMACIST: Dispense the accompanying Medication Guide to each patient.


SRC: NLM .
