Genvoya
Generic name: cobicistat, elvitegravir, emtricitabine, and tenofovir
Drug class: Antiviral combinations
Medically reviewed by A Ras MD.
What is Genvoya?
Genvoya is a prescription medicine that is used without other antiviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) in adults and children who weigh at least 55 pounds (25 kg) who have not received anti-HIV-1 medicines in the past, or to replace their current anti-HIV-1 medicines for people whose healthcare provider determines that they meet certain requirements.
HIV-1 is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
Genvoya contains the prescription medicines elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide.
It is not known if Genvoya is safe and effective in children who weigh less than 55 pounds (25 kg).
Description
GENVOYA (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) is a fixed-dose combination tablet containing elvitegravir (EVG), cobicistat (COBI), emtricitabine (FTC), and tenofovir alafenamide (TAF) for oral administration.
- EVG is an HIV-1 integrase strand transfer inhibitor.
- COBI is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
- FTC, a synthetic nucleoside analog of cytidine, is an HIV nucleoside analog reverse transcriptase inhibitor (HIV NRTI).
- TAF, an HIV NRTI, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
Each tablet contains 150 mg of EVG, 150 mg of COBI, 200 mg of FTC, and 10 mg of TAF (equivalent to 11.2 mg of tenofovir alafenamide fumarate). The tablets include the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium lauryl sulfate. The tablets are film-coated with a coating material containing FD&C Blue No. 2/indigo carmine aluminum lake, iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Elvitegravir: The chemical name of elvitegravir is 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
It has a molecular formula of C23H23ClFNO5 and a molecular weight of 447.88. It has the following structural formula:
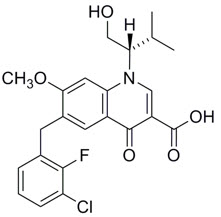
Elvitegravir is a white to pale yellow powder with a solubility of less than 0.3 micrograms per mL in water at 20 °C.
Cobicistat: The chemical name for cobicistat is 2,7,10,12-tetraazatridecanoic acid, 12-methyl-13-[2-(1-methylethyl)-4-thiazolyl]-9-[2-(4-morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl ester, (3R,6R,9S)-.
It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.02. It has the following structural formula:
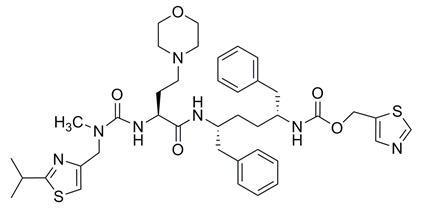
Cobicistat is adsorbed onto silicon dioxide. Cobicistat on silicon dioxide drug substance is a white to pale yellow powder with a solubility of 0.1 mg per mL in water at 20 °C.
Emtricitabine: The chemical name of emtricitabine is 4-amino-5-fluoro-1-(2R-hydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one. Emtricitabine is the (-)-enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5 position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
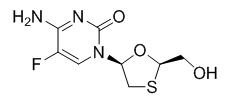
Emtricitabine is a white to off-white powder with a solubility of approximately 112 mg per mL in water at 25 °C.
Tenofovir alafenamide (TAF): The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1).
It has an empirical formula of C21H29O5N6P∙½(C4H4O4) and a formula weight of 534.5. It has the following structural formula:
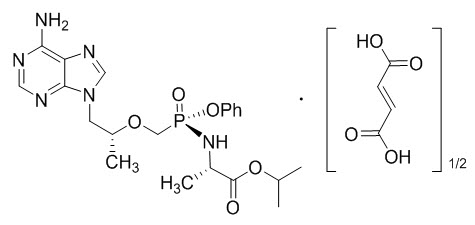
Tenofovir alafenamide fumarate is a white to off-white or tan powder with a solubility of 4.7 mg per mL in water at 20 °C.
What is the most important information I should know about Genvoya?
Genvoya can cause serious side effects, including:
- Worsening of Hepatitis B infection. If you have hepatitis B virus (HBV) infection and take Genvoya, your HBV may get worse (flare-up) if you stop taking Genvoya. A “flare-up” is when your HBV infection suddenly returns in a worse way than before.
- Do not run out of Genvoya. Refill your prescription or talk to your healthcare provider before your Genvoya is all gone.
- Do not stop taking Genvoya without first talking to your healthcare provider.
- If you stop taking Genvoya, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking Genvoya.
For more information about side effects, see “What are the possible side effects of Genvoya?”
Who should not take Genvoya?
Do not take Genvoya if you also take a medicine that contains:
- alfuzosin hydrochloride
- carbamazepine
- cisapride
- ergot-containing medicines, including:
- dihydroergotamine mesylate
- ergotamine tartrate
- methylergonovine maleate
- lomitapide
- lovastatin
- lurasidone
- midazolam, when taken by mouth
- phenobarbital
- phenytoin
- pimozide
- rifampin
- sildenafil, when used for treating the lung problem, pulmonary arterial hypertension
- simvastatin
- triazolam
- St. John’s wort (Hypericum perforatum) or a product that contains St. John’s wort.
What should I tell my healthcare provider before taking Genvoya?
Before taking Genvoya, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems, including hepatitis B infection
- have kidney problems
- are pregnant or plan to become pregnant.
- It is not known if Genvoya can harm your unborn baby.
- Genvoya should not be used during pregnancy because you may not have enough Genvoya in your body during pregnancy.
- Tell your healthcare provider if you become pregnant during treatment with Genvoya. Your healthcare provider may prescribe different medicines if you become pregnant while taking Genvoya.
- Pregnancy Registry: There is a pregnancy registry for women who take antiretroviral medicines during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk with your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take Genvoya.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- At least one of the medicines in Genvoya can pass to your baby in your breast milk. It is not known if the other medicines in Genvoya can pass into your breast milk.
Talk with your healthcare provider about the best way to feed your baby during treatment with Genvoya.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines may interact with Genvoya. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with Genvoya.
- Do not start a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Genvoya with other medicines.
How should I take Genvoya?
- Take Genvoya exactly as your healthcare provider tells you to take it. Genvoya is taken by itself (not with other HIV-1 medicines) to treat HIV-1 infection.
- Take Genvoya 1 time each day with food.
- If you are on dialysis, take your daily dose of Genvoya following dialysis.
- Do not change your dose or stop taking Genvoya without first talking with your healthcare provider. Stay under a healthcare provider’s care during treatment with Genvoya.
- If you need to take a medicine for indigestion (antacid) that contains aluminum hydroxide, magnesium hydroxide, or calcium carbonate during treatment with Genvoya, take it at least 2 hours before or after you take Genvoya.
- Do not miss a dose of Genvoya.
- When your Genvoya supply starts to run low, get more from your healthcare provider or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to Genvoya and become harder to treat.
- If you take too much Genvoya, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Genvoya?
Genvoya may cause serious side effects, including:
- See “What is the most important information I should know about Genvoya?”
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having any new symptoms after starting your HIV-1 medicine.
- New or worse kidney problems, including kidney failure. Your healthcare provider should do blood and urine tests to check your kidneys when starting and during treatment with Genvoya. Your healthcare provider may tell you to stop taking Genvoya if you develop new or worse kidney problems.
- Too much lactic acid in your blood (lactic acidosis). Too much lactic acid is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms:
- Severe liver problems. In rare cases, severe liver problems can happen that can lead to death. Tell your healthcare provider right away if you get these symptoms:
- skin or the white part of your eyes turns yellow,
- dark “tea-colored” urine, light-colored stools,
- loss of appetite for several days or longer,
- nausea, or stomach-area pain.
The most common side effect of Genvoya is nausea.
These are not all the possible side effects of Genvoya.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Genvoya
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Genvoya for a condition for which it was not prescribed. Do not give Genvoya to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Genvoya that is written for health professionals.
For more information, call 1-800-445-3235 or go to www.Genvoya.com.
How should I store Genvoya?
- Store Genvoya below 86°F (30°C).
- Keep Genvoya in its original container.
- Keep the container tightly closed.
Keep Genvoya and all medicines out of reach of children.
What are the ingredients in Genvoya?
Active ingredients: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide
Inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium lauryl sulfate. The tablets are film-coated with a coating material containing FD&C Blue No. 2/indigo carmine aluminum lake, iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Label;
PRINCIPAL DISPLAY PANEL – 30 TABLET BOTTLE LABEL
- NDC 61958-1901-1
30 tablets - Genvoya®
(elvitegravir, cobicistat,
emtricitabine, and tenofovir
alafenamide) Tablets
150 mg/150 mg/200 mg/10 mg - Note to pharmacist:
Do not cover ALERT box with pharmacy label. - ALERT: Find out about medicines that
should NOT be taken with Genvoya®

SRC: NLM .
