Gavreto
Generic name: pralsetinib
Drug class: Multikinase inhibitors
Medically reviewed by A Ras MD.
What is Gavreto?
Gavreto is a prescription medicine used to treat certain cancers caused by abnormal rearranged during transfection (RET) genes in adults with non-small cell lung cancer (NSCLC) that has spread, adults and children 12 years of age and older with advanced medullary thyroid cancer (MTC) or MTC that has spread who require a medicine by mouth or injection (systemic therapy).
IT is also used to treat adults and children 12 years of age and older with advanced thyroid cancer or thyroid cancer that has spread who require a medicine by mouth or injection (systemic therapy) and who have received radioactive iodine and it did not work or is no longer working.
Your healthcare provider will perform a test to make sure that Gavreto is right for you.
It is not known if Gavreto is safe and effective in children younger than 12 years of age
Description
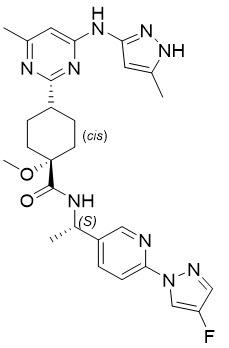
Pralsetinib is an oral receptor tyrosine kinase inhibitor. The chemical name for pralsetinib is (cis)-N-((S)-1-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)ethyl)-1-methoxy-4-(4-methyl-6-(5-methyl-1H-pyrazol-3-ylamino)pyrimidin-2-yl)cyclohexanecarboxamide. The molecular formula for pralsetinib is C27H32FN9O2, and the molecular weight is 533.61 g/mol. Pralsetinib has the following structure:
The solubility of pralsetinib in aqueous media decreases over the range pH 1.99 to pH 7.64 from 0.880 mg/mL to < 0.001 mg/mL, indicating a decrease in solubility with increasing pH.
GAVRETO (pralsetinib) is supplied for oral use as immediate release hydroxypropyl methylcellulose (HPMC) hard capsules containing 100 mg pralsetinib. The capsules also contain inactive ingredients:
citric acid, hydroxypropyl methylcellulose (HPMC), magnesium stearate, microcrystalline cellulose (MCC), pregelatinized starch and sodium bicarbonate. The capsule shell consists of FD&C Blue #1 (Brilliant Blue FCF), hypromellose and titanium dioxide. The white printing ink contains butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, strong ammonia solution and titanium dioxide.
Mechanism of Action
Pralsetinib is a kinase inhibitor of wild-type RET and oncogenic RET fusions (CCDC6-RET) and mutations (RET V804L, RET V804M and RET M918T) with half maximal inhibitory concentrations (IC50s) less than 0.5 nM. In purified enzyme assays, pralsetinib inhibited DDR1, TRKC, FLT3, JAK1-2, TRKA, VEGFR2, PDGFRB, and FGFR1 at higher concentrations that were still clinically achievable at Cmax. In cellular assays, pralsetinib inhibited RET at approximately 14-, 40-, and 12-fold lower concentrations than VEGFR2, FGFR2, and JAK2, respectively.
Certain RET fusion proteins and activating point mutations can drive tumorigenic potential through hyperactivation of downstream signaling pathways leading to uncontrolled cell proliferation. Pralsetinib exhibited anti-tumor activity in cultured cells and animal tumor implantation models harboring oncogenic RET fusions or mutations including KIF5B-RET, CCDC6-RET, RET M918T, RET C634W, RET V804E, RET V804L and RET V804M. In addition, pralsetinib prolonged survival in mice implanted intracranially with tumor models expressing KIF5B-RET or CCDC6-RET.
What should I tell my healthcare provider before taking Gavreto?
Before taking Gavreto, tell your healthcare provider about all of your medical conditions, including if you:
- have lung or breathing problems other than lung cancer
- have high blood pressure
- have bleeding problems
- plan to have surgery. You should stop taking Gavreto at least 5 days before your planned surgery. See “What are the possible side effects of Gavreto?”
- are pregnant or plan to become pregnant. Gavreto can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider will do a pregnancy test before you start treatment with Gavreto.
- You should use an effective form of non-hormonal birth control (contraception) during treatment and for 2 weeks after your final dose of Gavreto.
- Birth control methods that contain hormones (such as birth control pills, injections or transdermal system patches) may not work as well during treatment with Gavreto.
- Talk to your healthcare provider about birth control methods that may be right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Gavreto.
Males with female partners who are able to become pregnant:
- You should use effective birth control (contraception) during treatment and for 1 week after your final dose of Gavreto.
- are breastfeeding or plan to breastfeed. It is not known if Gavreto passes into your breast milk. Do not breastfeed during treatment and for 1 week after your final dose of Gavreto.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Gavreto may affect the way other medicines work, and other medicines may affect how Gavreto works.
How should I take Gavreto?
- Take Gavreto exactly as your healthcare provider tells you to take it.
- Take your prescribed dose of Gavreto 1 time each day.
- Take Gavreto on an empty stomach. Do not eat for at least 2 hours before and at least 1 hour after taking Gavreto.
- Do not change your dose or stop taking Gavreto unless your healthcare provider tells you to.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Gavreto if you develop side effects.
- If you miss a dose of Gavreto, take it as soon as possible on the same day. Then take your next dose of Gavreto at your regular time the next day.
- If you vomit after taking a dose of Gavreto, do not take an extra dose. Take your next dose of Gavreto at your regular time the next day.
What are the possible side effects of Gavreto?
Gavreto may cause serious side effects, including:
- Lung problems. Gavreto may cause severe or life-threatening inflammation of the lungs during treatment, that can lead to death. Tell your healthcare provider right away if you have any new or worsening symptoms, including:
- shortness of breath
- cough
- fever
- High blood pressure (hypertension). High blood pressure is common with Gavreto and may sometimes be severe. You should check your blood pressure regularly during treatment with Gavreto. Tell your healthcare provider if you have increased blood pressure readings or get any symptoms of high blood pressure, including:
- confusion
- headaches
- shortness of breath
- dizziness
- chest pain
- Liver problems. Liver problems (increased liver function blood test results) can happen during treatment with Gavreto and may sometimes be serious. Your healthcare provider will do blood tests before and during treatment with Gavreto to check you for liver problems. Tell your healthcare provider right away if you get any signs or symptoms of liver problem during treatment, including:
- Bleeding problems. Gavreto can cause bleeding which can be serious and cause death. Tell your healthcare provider if you have any signs or symptoms of bleeding during treatment, including:
- vomiting blood or if your vomit looks like coffee-grounds
- pink or brown urine
- red or black (looks like tar) stools
- coughing up blood or blood clots
- unusual bleeding or bruising of your skin
- menstrual bleeding that is heavier than normal
- unusual vaginal bleeding
- nose bleeds that happen often
- drowsiness or difficulty being awakened
- confusion
- headache
- change in speech
- Tumor lysis syndrome (TLS). TLS is caused by a fast breakdown of cancer cells. TLS can cause you to have kidney failure and the need for dialysis treatment, an abnormal heartbeat, and may sometimes lead to hospitalization. Your healthcare provider may do blood tests to check you for TLS. You should stay well hydrated during treatment with Gavreto. Call your healthcare provider or get emergency medical help right away if you develop any of these symptoms during treatment with Gavreto:
- nausea
- vomiting
- weakness
- swelling
- shortness of breath
- muscle cramps
- seizures
- Risk of wound healing problems. Wounds may not heal properly during treatment with Gavreto. Tell your healthcare provider if you plan to have any surgery before or during treatment with Gavreto.
- You should not take Gavreto for at least 5 days before surgery.
- Your healthcare provider should tell you when you may start taking Gavreto again after surgery.
The most common side effects of Gavreto include:
- tiredness
- constipation
- muscle and joint pain
- high blood pressure
- decreased white blood cell and red blood cell counts
- decreased levels of phosphate in the blood
- decreased levels of body salt (sodium) in the blood
- decreased levels of calcium in the blood
- abnormal liver function blood tests
Gavreto may affect fertility in males and females, which may affect your ability to have children. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Gavreto.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Gavreto
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Gavreto for a condition for which it was not prescribed. Do not give Gavreto to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Gavreto that is written for health professionals.
How should I store Gavreto?
- Store Gavreto at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect Gavreto from moisture.
Keep Gavreto and all medicines out of the reach of children.
What are the ingredients in Gavreto?
Active ingredient: pralsetinib
Inactive ingredients: citric acid, hydroxypropyl methylcellulose (HPMC), magnesium stearate, microcrystalline cellulose (MCC), pregelatinized starch and sodium bicarbonate.
Capsule shell: FD&C Blue #1 (Brilliant Blue FCF), hypromellose and titanium dioxide.
White printing ink: butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, strong ammonia solution and titanium dioxide.
Label
PRINCIPAL DISPLAY PANEL – 100 MG CAPSULE BOTTLE LABEL
- NDC 50242-210-60
- Gavreto®
(pralsetinib)
capsules - 100 mg
- For Oral Use
- Rx only
- 60 Capsules
- Genentech
blueprint™
MEDICINES - 10242131

SRC: NLM .
