Forteo
Generic name: teriparatide
Drug class: Parathyroid hormone and analogs
Medically reviewed by A Ras MD.
What is Forteo?
Forteo is a prescription medicine that is like a hormone made by the body called parathyroid hormone or PTH. Forteo may help to form new bone, increase bone mineral density and bone strength. Forteo can lessen the number of fractures of the spine and other bones in postmenopausal women with osteoporosis.
Forteo is used in both men and postmenopausal women with osteoporosis who are at high risk for having fractures. Forteo can be used by people who have had a fracture related to osteoporosis, or who have several risk factors for fracture, or who can not use other osteoporosis treatments.
Forteo is used in both men and women with osteoporosis due to use of glucocorticoid medicines, such as prednisone, for several months, who are at high risk for having broken bones (fractures). These include men and women with either a history of broken bones, who have several risk factors for fracture, or who can not use other osteoporosis treatments.
It is not known if Forteo is safe and effective in children.
Forteo should not be used in children and young adults whose bones are still growing.
Description
FORTEO (teriparatide injection) is a recombinant human parathyroid hormone analog (PTH 1-34). It has an identical sequence to the 34 N-terminal amino acids (the biologically active region) of the 84-amino acid human parathyroid hormone.
The molecular formula of teriparatide is C181H291N55O51S2 and molecular weight is 4117.8 daltons. Its amino acid sequence is shown below:
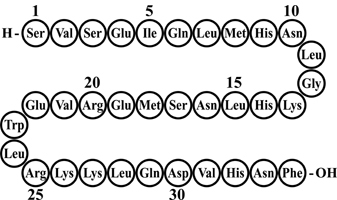
Teriparatide is manufactured using a strain of Escherichia coli modified by recombinant DNA technology.
FORTEO is supplied as a sterile, colorless, clear, isotonic solution in a glass cartridge which is pre-assembled into a single-patient-use delivery device (pen) for subcutaneous injection. Each delivery device (pen) is filled with 2.7 mL to deliver 2.4 mL. Each mL contains 250 mcg of teriparatide (as a free base), 0.41 mg of glacial acetic acid, 0.1 mg of sodium acetate (anhydrous), 45.4 mg of mannitol, 3 mg of Metacresol, and Water for Injection. In addition, hydrochloric acid solution 10% and/or sodium hydroxide solution 10% may have been added to adjust the pH to 4.
Each prefilled delivery device (pen) delivers 20 mcg of teriparatide per dose for up to 28 days.
Mechanism of Action
Endogenous 84-amino acid parathyroid hormone (PTH) is the primary regulator of calcium and phosphate metabolism in bone and kidney. Physiological actions of PTH include regulation of bone metabolism, renal tubular reabsorption of calcium and phosphate, and intestinal calcium absorption. The biological actions of PTH and teriparatide are mediated through binding to specific high-affinity cell-surface receptors. Teriparatide and the 34 N-terminal amino acids of PTH bind to these receptors with the same affinity and have the same physiological actions on bone and kidney. Teriparatide is not expected to accumulate in bone or other tissues.
The skeletal effects of teriparatide depend upon the pattern of systemic exposure. Once-daily administration of teriparatide stimulates new bone formation on trabecular and cortical (periosteal and/or endosteal) bone surfaces by preferential stimulation of osteoblastic activity over osteoclastic activity. In monkey studies, teriparatide improved trabecular microarchitecture and increased bone mass and strength by stimulating new bone formation in both cancellous and cortical bone. In humans, the anabolic effects of teriparatide manifest as an increase in skeletal mass, an increase in markers of bone formation and resorption, and an increase in bone strength. By contrast, continuous excess of endogenous PTH, as occurs in hyperparathyroidism, may be detrimental to the skeleton because bone resorption may be stimulated more than bone formation.
What is the most important information I should know about Forteo?
- Possible bone cancer. During drug testing, the medicine in Forteo caused some rats to develop a bone cancer called osteosarcoma. In people, osteosarcoma is a serious but rare cancer. Osteosarcoma has rarely been reported in people who took Forteo. It is not known if people who take Forteo have a higher chance of getting osteosarcoma.
- You should not take Forteo for more than 2 years over your lifetime.
- There is a voluntary Patient Registry for people who take Forteo. The purpose of the registry is to collect information about the possible risk of osteosarcoma in people who take Forteo. For information about how to sign up for this patient registry, call 1-866-382-6813 or go to www.Forteoregistry.rti.org.
Who should not use Forteo?
Do not use Forteo if you:
- are allergic to any of the ingredients in Forteo. See the end of this Medication Guide for a complete list of the ingredients in Forteo.
What should I tell my healthcare provider before taking Forteo?
Before you take Forteo, tell your healthcare provider if you:
- have the condition listed in the section “Who should not use Forteo?”
- have Paget’s disease or other bone disease
- have cancer in your bones
- have trouble injecting yourself and do not have someone who can help you
- are a child or young adult whose bones are still growing
- have or have had kidney stones
- have had radiation therapy
- have or had too much calcium in your blood
- have any other medical conditions
- are pregnant or thinking about becoming pregnant. It is not known if Forteo will harm your unborn baby.
- are breast-feeding or plan to breast-feed. You should not breast-feed while taking Forteo.
Tell your healthcare provider about all the medicines you take including prescription and non-prescription medicines, vitamins, and herbal supplements. Your healthcare provider needs this information to help keep you from taking Forteo with other medicines that may harm you.
- Especially tell your doctor if you take medicines that contain digoxin (Digoxin, Lanoxicaps, Lanoxin).
How should I take Forteo?
- Inject Forteo one time each day in your thigh or abdomen (lower stomach area). Talk to a healthcare provider about how to rotate injection sites.
- Before you try to inject Forteo yourself, a healthcare provider should teach you how to use the Forteo delivery device to give your injection the right way.
- Read the detailed User Manual that comes with your medication.
- You can take Forteo with or without food or drink.
- The Forteo delivery device has enough medicine for 28 days. It is set to give a 20 microgram dose of medicine each day. Do not inject all the medicine in the Forteo delivery device at any one time.
- Do not transfer the medicine from the Forteo delivery device to a syringe. This can result in taking the wrong dose of Forteo. If you do not have pen needles to use with your Forteo delivery device, talk with your healthcare provider.
- Forteo should look clear and colorless. Do not use Forteo if it has particles in it, or if it is cloudy or colored.
- Inject Forteo right away after you take the delivery device out of the refrigerator.
- After each use, safely remove the needle, recap the delivery device, and put it back in the refrigerator right away.
- You can take Forteo at any time of the day. To help you remember to take Forteo, take it at about the same time each day.
- If you forget or cannot take Forteo at your usual time, take it as soon as you can on that day. Do not take more than one injection in the same day.
- If you take more Forteo than prescribed, call your healthcare provider. If you take too much Forteo, you may have nausea, vomiting, weakness, or dizziness.
Follow your healthcare provider’s instructions about other ways you can help your osteoporosis, such as exercise, diet, and reducing or stopping your use of tobacco and alcohol. If your healthcare provider recommends calcium and vitamin D supplements, you can take them at the same time you take Forteo.
What are the possible side effects of Forteo?
Forteo can cause serious side effects including:
- See “What is the most important information I should know about Forteo?”
- Decrease in blood pressure when you change positions. Some people feel dizzy, get a fast heartbeat, or feel faint right after the first few doses. This usually happens within 4 hours of taking Forteo and goes away within a few hours. For the first few doses, take your injections of Forteo in a place where you can sit or lie down right away if you get these symptoms. If your symptoms get worse or do not go away, stop taking Forteo and call your healthcare provider.
- Increased calcium in your blood. Tell your healthcare provider if you have nausea, vomiting, constipation, low energy, or muscle weakness. These may be signs there is too much calcium in your blood.
Common side effects of Forteo include:
- nausea
- joint aches
- pain
Your healthcare provider may take samples of blood and urine during treatment to check your response to Forteo. Also, your healthcare provider may ask you to have follow-up tests of bone mineral density.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Forteo. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Forteo
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Forteo for a condition for which it was not prescribed. Do not give Forteo to other people, even if they have the same condition you have.
This Medication Guide summarizes the most important information about Forteo. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Forteo that is written for healthcare professionals. For more information, go to www.Forteo.com or call Lilly at 1-866-436-7836.
How should I store Forteo?
- Keep your Forteo delivery device in the refrigerator between 36° to 46°F (2° to 8°C).
- Do not freeze the Forteo delivery device. Do not use Forteo if it has been frozen.
- Do not use Forteo after the expiration date printed on the delivery device and packaging.
- Throw away the Forteo delivery device after 28 days even if it has medicine in it (see the User Manual).
Keep Forteo and all medicines out of the reach of children.
What are the ingredients in Forteo?
Active ingredient: teriparatide
Inactive ingredients: glacial acetic acid, sodium acetate (anhydrous), mannitol, metacresol, and water for injection. In addition, hydrochloric acid solution 10% and/or sodium hydroxide solution 10% may have been added to adjust the product to pH 4.
Label
PACKAGE LABEL – FORTEO 20 mcg per dose, 2.4 mL
- Do NOT transfer contents to a syringe
- ATTENTION PHARMACIST: Medication Guide and device User Manual for patient inside carton
- NDC 0002-8400-01
- MS8400
- FORTEO®
- teriparatide injection
- 20 mcg per dose (given once daily subcutaneously)
- Each single-patient-use prefilled pen will deliver 28 subcutaneous doses.
- 600 mcg/ 2.4 mL (250 mcg/mL)
- For Single-Patient-Use Only
- REFRIGERATE / DO NOT FREEZE
- For subcutaneous use / Rx only
- Needles not included
- Becton, Dickinson and Company pen needles are recommended for use with this device
- www.forteo.com
- Lilly

